��Ŀ����
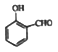
����Ŀ���������Ƿ������Ȼ��뱽��ֱ��������һ���л��ͨ���÷��������������Ʊ�����Ӧԭ�����£�
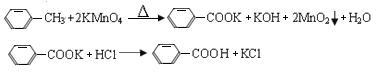
��Ӧ�Լ������������������
���� | ��Է������� | ��״ | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g/cm3�� | ˮ�е��ܽ��� |
�ױ� | 92 | ��ɫҺ����ȼ�ӷ� | -95 | 110.6 | 0.8669 | ���� |
������ | 122 | ��ɫƬ״����״���� | 122.4 | 248 | 1.2659 | �� |
��Ҫʵ��װ�ú��������£�
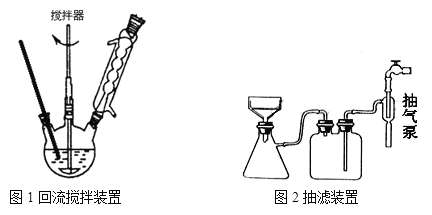
ʵ�鷽����һ�����ļױ���KMnO4��Һ����ͼl װ���У���90��ʱ����Ӧһ��ʱ���
ֹͣ��Ӧ�����������̷���������Ტ����δ��Ӧ�ļױ���
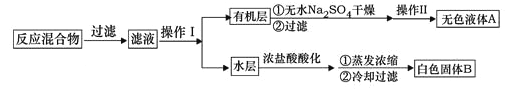
��1����ɫ����B����Ҫ�ɷֵķ���ʽΪ________��������Ϊ________��
��2�������Һ����ɫ���������������أ������ӷ���ʽ��ʾ��ԭ��__________��
��3�����й�����������װ����ʹ����ȷ����__________��
A�����˿��Լӿ�����ٶȣ��õ��ϸ���ij���
B����װ�綯������ʱ���������¶˲�����������ƿ�ס��¶ȼƵȽӴ�
C����ͼ ��������װ��Ӧ����ֱ�Ӽ��ȵķ���
D����������ˮ���������Ͻ��³�
��4����ȥ�����ڱ������еļױ�Ӧ�ȼ���______����Һ��Ȼ������ˮ���м���______�����ˣ�ϴ�ӣ����T�ɵõ������ᡣ
��5�����Ȳⶨ����ȡ2.440g��Ʒ�����100mL��Һ��ȡ����25.00mL ��Һ�����еζ�������KOH���ʵ���Ϊ4.5��10-3mol����Ʒ�б�������������Ϊ_______��
���𰸡� C7H6O2 ���� 2MnO4 - +5HSO3- + H += 2 Mn2+ +5SO42- + 3H2O AB NaOH��Һ �����Ũ�����ữ 90%
��������(1)��ɫ�����DZ����ᣬ����ʽΪC7H6O2���л��������ʻ����ҷе㲻ͬ�����Կ��Բ����������룬�����IIΪ����
(2)�����Һ����ɫ��˵��������ع�����Ҫ�ȼ���������أ���ȥδ��Ӧ�ĸ�����أ�������Ӧ�����ӷ���ʽΪ2MnO4 - +5HSO3- + H += 2 Mn2+ +5SO42- + 3H2O��
(3)A������ʱ��ƿ��ѹǿ��С�����Լӿ�����ٶȣ��õ��ϸ���ij�������A��ȷ��B��Ϊ�˷�ֹ������¶˴�������ƿ���¶ȼƣ���˲��������ǽӴ��������ڽ���ʱ��������¶˲�����������ƿ�ס��¶ȼƵȽӴ�����B��ȷ��C����ˮԡ���ȱ��ڿ����¶Ⱥ�ʹ�������Ⱦ��ȣ�ͼ1��������װ��Ӧ����ˮԡ���ȵķ�������C����D����������ˮ�������������������෴������������ˮ���������½��ϳ�����D���ʴ�ΪABD��
(4)��ȥ�����ڱ������еļױ�Ӧ�ȼ��룬Ӧ���ȼ�NaOH��Һ���ױ���NaOH����Ӧ����������NaOH��Ӧ���ɱ������ƣ���Һ������������Һ�м���������Ƶñ����
(5)�豽��������ʵ���Ũ��Ϊx����25mL��������Һ�б���������ʵ���Ϊ0.025xmol��
C6H5COOH+KOH��C6H5COOK+H2O
1mol 1mol
0.025xmol 4.50��10-3mol
1mol��1mol=0.025xmol��4.50��10-3mol
x=![]() =0.18��
=0.18��
��100mL����������������=0.18mol/L��0.1L��122g/mol=2.196g������������=![]() =90%��
=90%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij��ȤС����Ƴ���ͼ��ʾװ�����Ľ��̲��С�ͭ�����ᷴӦ��ʵ�飬��̽����ѧʵ�����ɫ����
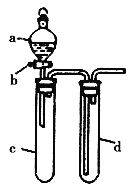
(1)ʵ��ǰ���رջ���b���Թ�d�м�ˮ����û�����ܿڣ������Թ�c��d�Ľ���������c����Ŀ����_____________________��
(2)��d�м�����NaOH��Һ��c�з�һС��ͭƬ���ɷ�Һ©��a��c�м���2 mLŨ���ᣬc�з�Ӧ�Ļ�ѧ����ʽ��______________________________________________��
(3)�±�����ȡ����ͭ�����ַ�������������ɫ��ѧ�������ѷ�����____________��
���� | ��Ӧ�� |
�� | Cu��ŨHNO3 |
�� | Cu��ϡHNO3 |
�� | Cu��O2��ϡHNO3 |
(4)��С�黹������װ�ý���ʵ��֤�����ԣ�HCl��H2CO3��H2SiO3�����Һ©��a�м�����Լ���____________��c�м�����Լ���____________��d�м�����Լ���____________��ʵ������Ϊ____________________________________________����ͬѧ��Ϊ����ʵ��װ���Բ���֤���������ۣ��Ľ��Ĵ�ʩ��_________________________________________��