��Ŀ����
����Ŀ����������һ����ɫ���壬���������ࡢ�ƾ���ӡˢ��ҽҩ�ȡ���ˮ��Һ���峣����������ɫ�Լ�ƿ�С����������ȶ������ȷֽ����ɹ��塢NO2 �� O2 ��Ϊ����֤ AgNO3 ���ȷֽ�IJ��ijͬѧ���������ʵ�飬ʵ�����õ�װ����ͼ��ͼ�м��ȡ��г������Ⱦ���ȥ����

ʵ�鲽�裺
a���������Ӻ���AgNO3����֮ǰ���ر�K����Ӳ�ʲ�����A��
b����ȡ AgNO3 ����1.7g ����A�У���ͨһ��ʱ��N2���ٹر�K���þƾ��Ƽ���Ӳ�ʲ�����A��
c������Ʒ��ȫ�ֽ⣬װ��A��ȴ�����£����������ʣ����������Ϊ1.08 g��
��ش��������⣺
��1��ʹ�þƾ��ƶ�AgNO3������ȵIJ���������_________________��
��2������a�IJ���Ŀ����__________________��
��3������b����ͨһ��ʱ���N2��Ŀ����_____________________��
��4��װ��Cƿ��ʢװ��ҩƷ����Ϊ_____________���������ɣ���װ��D�пɹ۲쵽��ʵ������Ϊ__________________��
��5������ʵ������ͳ����������д����װ����AgNO3�������ȷֽ�Ļ�ѧ����ʽ__________________��
��6����ʵ�鰲ȫ�Ƕȿ��Ǹ�װ�ô���ȱ�ݣ�Ӧ��θĽ���__________________��
���𰸡���Ԥ���ټ��м��� ���װ��������(����װ���Ƿ�©��) ��װ������������(����Ҳ��)������Ӱ��ʵ���� �������Ƶķ�̪��Һ �ۺ�ɫ��dz 2AgNO3 ![]() 2Ag + 2NO2�� + O2�� ��A��B֮������һ��������װ��(��ȫƿ)
2Ag + 2NO2�� + O2�� ��A��B֮������һ��������װ��(��ȫƿ)
��������
���������ȶ������ȷֽ����ɹ��塢NO2��O2��Ϊ����֤ AgNO3 ���ȷֽ�IJ����Ҫ�õ����ų�װ���ڵĿ���������ͼʾ��װ��B������������Ϊ���������ɵĶ���������װ��D�������������ɵ������ģ������Ҫ��װ��C����NO2��û��ȫ����B��NaOH���գ�װ��E��Ϊ�˷�ֹ����������װ��D��Ӱ��ʵ�������ݴ˷������
(1)Ϊ��ֹӲ�ʲ��������Ȳ�����ը�ѣ�ʹ�þƾ��ƶ�AgNO3�������ʱ��Ҫ�ȸ��Թ�Ԥ�ȣ��ټ��м��ȣ��ʴ�Ϊ����Ԥ���ټ��м��ȣ�
(2)�������Ӻ���AgNO3����֮ǰ���ر�K����Ӳ�ʲ�����A�����B��C��D��E�е��ܿ������ݣ���ȴ�����γ�һ��ˮ����˵��װ�������Ժã�������a�IJ���Ŀ���Ǽ��װ�õ������ԣ��ʴ�Ϊ�����װ�������ԣ�
(3)����b����ͨ��һ��ʱ��N2�����Գ�ȥװ���ڵĿ�������ֹ����������Ӱ��ֽ�����������������ʴ�Ϊ���������ڲ����ڵ������ų�������Ӱ��ʵ������
(4)����װ��B��ʢ�ŵ�NaOH���Գ�ȥ���������е�NO2��Ϊ�˲�Ӱ��D�������ļ��飬��Ҫ��װ��C����NO2��û��ȫ����B��NaOH���գ�����ѡ��NaOH��̪��Һ������Һ��ɫ���䣬���ʾNO2��B��NaOH������ȫ��װ��D��Na2SO3����������ΪNa2SO4����Լ�������Һ��ɫ��dz���ʴ�Ϊ��NaOH��̪��Һ����Һ��ɫ��dz��
(5)1.7g AgNO3 �����ʵ���=![]() =0.01mol��ʣ������к���0.01molAg������Ϊ1.08 g��˵��ʣ�����Ϊ����ͬʱ���ɶ������������������AgNO3�������ȷֽ�Ļ�ѧ����ʽΪ2AgNO3
=0.01mol��ʣ������к���0.01molAg������Ϊ1.08 g��˵��ʣ�����Ϊ����ͬʱ���ɶ������������������AgNO3�������ȷֽ�Ļ�ѧ����ʽΪ2AgNO3 ![]() 2Ag + 2NO2�� + O2�����ʴ�Ϊ��2AgNO3
2Ag + 2NO2�� + O2�����ʴ�Ϊ��2AgNO3 ![]() 2Ag + 2NO2�� + O2����
2Ag + 2NO2�� + O2����
(6)NO2��������NaOH��Һ��Ϊ�˷�ֹ������Ӧ��A��B֮����������װ�ã�����B����Һ������װ��A�У��ʴ�Ϊ��Ӧ��A��B֮����������װ�á�

����Ŀ���״�����Ҫ�Ļ���ԭ�ϡ��ڴ����������£����úϳ���(��Ҫ�ɷ�ΪCO��CO2��H2)�ϳɼ״�����Ҫ��ѧ��Ӧ���£�
I.CO+2H2![]() CH3OH II.CO2+3H2
CH3OH II.CO2+3H2![]() CH3OH+H2O
CH3OH+H2O
��.CO2+H2![]() CO+H2O��
CO+H2O��
��ش��������⣺
(1)��֪�������ʵı�ȼ�������±���
���� | CO(g) | H2(g) | CH3OH(l) |
ȼ����(kJ��mol1) | 283.0 | 285.8 | 726.51 |
����д25�桢101kPa����ʱCOȼ���ȵ��Ȼ�ѧ����ʽ______________________��
�ڼ���25�桢101kPa����ʱ��Ӧ�����H=_____kJ��mol1 ��
(2)ֱ�Ӽ״�ȼ�ϵ��(Direct Methanol Fuel Cell)�������ӽ���Ĥȼ�ϵ�أ��乤��ԭ����ͼ��ʾ��
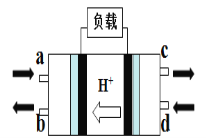
��c��������������________
�ڸ����ĵ缫��Ӧʽ��____________��
(3)��ͼ�Ǽ״�ȼ�ϵ�ع���ʾ��ͼ������A��B��D��Ϊʯī�缫��CΪͭ�缫������һ��ʱ��Ͽ�K����ʱA��B�����ϲ��������������ͬ��

������B���ĵ缫��Ӧʽ_______��
������A�������������ڱ���µ����________��
�۱�װ����Һ�н��������ӵ����ʵ�����ת�Ƶ��ӵ����ʵ����仯��ϵ��ͼ������߱�ʾ����____�ı仯����Ӧ������Ҫʹ��װ���н���������ǡ����ȫ��������Ҫ____ml 5mol/L NaOH��Һ��
����Ŀ��ʵ�������Ʊ����ȱ�����ķ�Ӧ�Լ�װ����ͼ��ʾ��
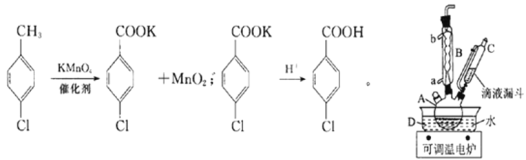
�����£����ʵ��й����ݺ����������ʾ��
�۵�/�� | �е�/�� | �ܶ�/g��cm��3 | ��ɫ | ˮ���� | |
���ȼױ� | 7.5 | 162 | 1.07 | ��ɫ | ���� |
���ȱ����� | 243 | 275 | 1.54 | ��ɫ | �� |
���ȱ������ | �����ε�ͨ�ԣ����ڿ������� | ||||
ʵ�鲽�裺�ڹ��Ϊ250mL������A�м���һ�����Ĵ���������KMnO4��100mLˮ����װ��װ�ã��ڵ�Һ©���м���6.00mL���ȼױ������¶�Ϊ93������ʱ����ε�����ȼױ��������¶���93�����ң���Ӧ2h�����ˣ�����������ˮϴ�ӣ�ʹϴ��Һ����Һ�ϲ�������ϡ�����ữ������Ũ������ȴ��Ȼ����ˣ�����������ˮ����ϴ�ӣ�����������������
��ش��������⣺
(1)����A������Ϊ______________________��
(2)����B�������ܣ�������Ҫ�����ǣ�________________��ʵ������У���ȴˮ��________�ڡ�
(3)ʵ����������ι��ˡ�ϴ�Ӳ�������һ�ι��˵������ɷ�Ϊ___________(�ѧʽ)��ϴ�Ӹ���������ˮ��Ŀ����_________________________________���ڶ��ι��˺�ϴ����������ˮ��Ŀ����______________________��
(4)���ˡ�ϴ�Ӳ��������õ���������___________(��ѡ����ĸ)��
a.�ձ� b.��Һ©�� c.��ƿ d.������
(5)��һ�ι��˺����Һ�м������ᣬ���ֵ�������___________��
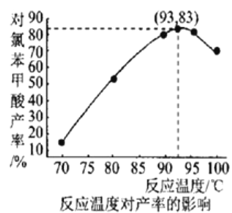
(6)��ͼ���¶ȶԶ��ȱ�������ʵ�Ӱ���ϵ������������õ��Ķ��ȱ����������Ϊ___________(����С�������λ)��