��Ŀ����
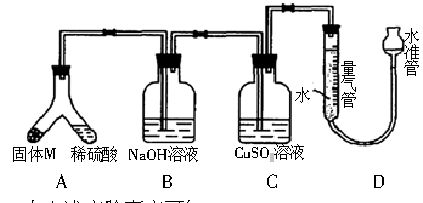
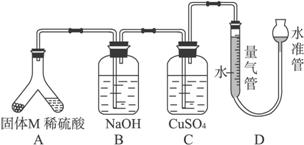
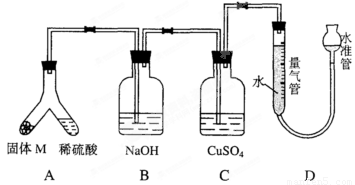
ij������ȤС��Ϊ��̽��ij�����Ͻ𣨺Ͻ�Ԫ��ΪMg���Ƿ���Ϲ��������������ҹ涨�������������ܵ���78%�����������ͼװ�ý���ʵ�顣
��1����μ����װ���Ƿ��ܷ�____________________��
��2������M������������Һ��Ӧ�����ӷ���ʽ_______��
��3����бAʹ����������Һ����������Ͻ��ĩ������M��ag��ַ�Ӧ������Ӧֹͣ��Ӧ�������������������ΪVmL��������ɱ�״���������������������Ϊ50mL�������M������Ӧ_________��
��4������װ���е�����������Һ�滻Ϊ���������ᣬ��Ӧֹͣ�����������������____���>������<������=���� VmL����a =38mg��V=44.8mL���úϽ�________������ϡ����������ϡ������ұ���
��5����һ��ȤС�����ø�װ�òⶨMg�����ԭ����������Ҫֱ�Ӳⶨ��������Ϊ______��
a������ϡ���ᣨŨ����֪������� b��þ�������� c�������ܶ���
���ⶨ���ƫС�����ܵ�ԭ����_____________��
a��þ����������Ĥδ���� b����ȡ����ʱˮ�ܵ�ˮ����������ܵ�ˮ��
c��δ����ȴ����ȡ�����ܶ��� d��װ��©��
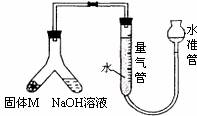
��1����װ��װ�ò����������ڼ���ˮ�������ƶ�ˮƿ����ˮƿ��Һ����������Һ��ά��һ���ĸ߶Ȳ˵��װ���ܷ�
��2��2OH- + 2Al + 2H2O===2AlO2-+ 3H2�� ��3����0.040g
��4��>������ ��5��bc c