��Ŀ����
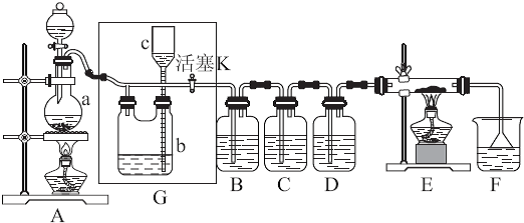
����ͼ��ʾװ�ÿ������һϵ��ʵ�飨ͼ�мг�װ������ȥ��
��ش��������⣺
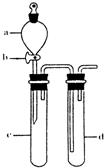
I����1������p��������
| B������� | �� | �� | �� |
| ��պ�Լ� | ʯ����Һ | Ʒ����Һ | ��ˮ����ɫ�� |
| ���� | ��ɫ | ||
| ����SO2������ | ˮ��Һ������ |
II������װ��A��Ũ������������ȡCl2���壬װ��B�е��Ĵ��������������´�������պ��FeCl2��Һ ��պ�е���KI��Һ ��պ��ʯ����Һ ��պ��Ʒ����Һ
��1��д���ٴ�������Ӧ�����ӷ���ʽ
��2����ʵ������У��۴��ܹ۲쵽������
��3��д��װ��C�С����ն������塱���õ��Լ�
��������1������p�������Ƿ�Һ©��������SO2������ˮ���������ᣨH2SO3����H2SO3�����ԣ�����SO2����Ư���ԣ���ʹƷ����Һ��ɫ��
���ݵ���ʹ������Һ������SO2���л�ԭ�ԣ������ˮ��Ӧ��ʹ��ɫ��ȥ��SO2���������ԣ����������ᷴӦ��
��2����Ͷ��������������ԭ��Ӧ��I2+SO2+2H2O�T4H++SO2-4+2I-��
II����1���������ӻᱻ��������Ϊ���������ӣ�
��2������Cl2����ˮ��Ӧ����HCl��HClO��HCl�������Ժ�HClO����ǿ�����Խ��
��3�������ж��������ŷ��ڻ����У���NaOH��Һ���������Ȼ�����������ƣ�
���ݵ���ʹ������Һ������SO2���л�ԭ�ԣ������ˮ��Ӧ��ʹ��ɫ��ȥ��SO2���������ԣ����������ᷴӦ��
��2����Ͷ��������������ԭ��Ӧ��I2+SO2+2H2O�T4H++SO2-4+2I-��
II����1���������ӻᱻ��������Ϊ���������ӣ�
��2������Cl2����ˮ��Ӧ����HCl��HClO��HCl�������Ժ�HClO����ǿ�����Խ��
��3�������ж��������ŷ��ڻ����У���NaOH��Һ���������Ȼ�����������ƣ�
����⣺��1������p�������Ƿ�Һ©��������SO2������ˮ���������ᣨH2SO3����H2SO3�����ԣ�����SO2����Ư���ԣ���ʹƷ����Һ��ɫ��
���ݵ���ʹ������Һ������SO2���л�ԭ�ԣ������ˮ��Ӧ��ʹ��ɫ��ȥ��
�ʴ�Ϊ����Һ©����
��2��SO2���ˮ����������ԭ��Ӧ��SO2+I2+2H2O=H2SO4+2HI����Һ��ɫ��ȥ��
�ʴ�Ϊ��I2+SO2+2H2O�T4H++SO2-4+2I-��
II����1���������ӻᱻ��������Ϊ���������ӣ���Ӧʵ����2Fe2++Cl2�T2Fe3++2Cl-���ʴ�Ϊ��2Fe2++Cl2�T2Fe3++2Cl-��
��2����Cl2����ˮ��Ӧ����HCl��HClO��HCl�������ԣ���ʹʯ����Һ��죬HClO����ǿ��������ʹ��ɫ��ȥ���ʴ�Ϊ��������ɫ���ɫ������ɫ��
��3��������NaOH��Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ����ѧ��ӦΪ2NaOH+Cl2=NaCl+NaClO+H2O���ʴ�Ϊ���������ƣ�NaOH����Һ��
���ݵ���ʹ������Һ������SO2���л�ԭ�ԣ������ˮ��Ӧ��ʹ��ɫ��ȥ��
�ʴ�Ϊ����Һ©����
| ���� | ��� | ��ɫ | |
| ����SO2������ | Ư���� | ��ԭ�� |
�ʴ�Ϊ��I2+SO2+2H2O�T4H++SO2-4+2I-��
II����1���������ӻᱻ��������Ϊ���������ӣ���Ӧʵ����2Fe2++Cl2�T2Fe3++2Cl-���ʴ�Ϊ��2Fe2++Cl2�T2Fe3++2Cl-��
��2����Cl2����ˮ��Ӧ����HCl��HClO��HCl�������ԣ���ʹʯ����Һ��죬HClO����ǿ��������ʹ��ɫ��ȥ���ʴ�Ϊ��������ɫ���ɫ������ɫ��
��3��������NaOH��Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ����ѧ��ӦΪ2NaOH+Cl2=NaCl+NaClO+H2O���ʴ�Ϊ���������ƣ�NaOH����Һ��
������������ʵ����Ҫ�����������Ͷ�����������ʣ��ܹ�����ʵ�������ж����ʵ����ʣ����ӷ�Ӧ����������ж��ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
 SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��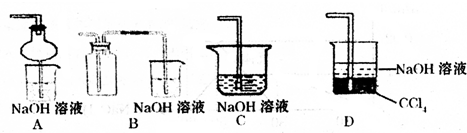
 SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��