��Ŀ����
��֪N2(g)��3H2(g)![]() 2NH3(g)������һ�ܱ������г���1molN2��3molH2���ڸ��¡���ѹ�ʹ������������·�����Ӧ�������й�˵����ȷ���ǣ� ��
2NH3(g)������һ�ܱ������г���1molN2��3molH2���ڸ��¡���ѹ�ʹ������������·�����Ӧ�������й�˵����ȷ���ǣ� ��
A�����տ�������2mol NH3
B���ﵽ��ѧƽ��״̬ʱ������Ӧ���淴Ӧ�����ʶ�Ϊ0
C���ﵽ��ѧƽ��״̬ʱ��������N2��H2��NH3�����ʵ���֮��Ϊ1��3��2
D���ﵽ��ѧƽ��״̬ʱ��N2��H2��NH3�����ʵ���Ũ�Ȳ��ٱ仯
D
����:��ѧƽ��״̬�����淴Ӧ������ȵ�һ����̬ƽ�⣬�����ʵ�Ũ�Ȳ��ٸı䣬�������ʼ�û��ȷ���ı�����ϵ�����Ը÷�Ӧ�������Խ��е��ף��������ɰ���С��2 mol��

��ϰ��ϵ�д�
�����Ŀ
��ѧ��һֱ�������о����¡���ѹ�¡��˹��̵������·���������ʵ�鱨�����ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ�����ɵ���Ҫ����ΪNH3����һ���о�NH3���������¶ȵĹ�ϵ������ʵ�����ݼ��±������ա�N2ѹ��1.0��105 Pa����Ӧʱ��3h����
��Ӧ�Ļ�ѧ����ʽ��N2��g��+3H2O��l��?2NH3��g��+
O2��g����H=+765.2kJ?mol-1
�ش��������⣺
��1���÷�Ӧ�ڽϵ��¶����ܷ��Է����У� ��
��2����323K��353K�����������������ٵ�ԭ�� ��
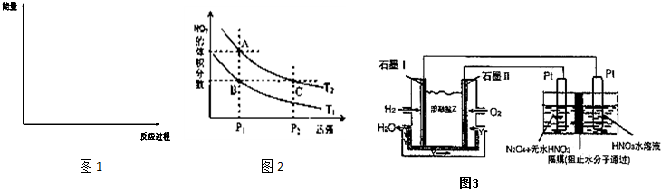
��3���뻭��������Ӧ���д�����������������·�Ӧ��������ϵ�����仯ʾ��ͼ1�������б�Ҫ��ע��
��4����ҵ�ϳɰ��ķ�ӦΪN2��g��+3H2��g��?2NH3��g���������ݻ�Ϊ2.0L���ܱ������г���0.60mol N2��g����1.60mol H2��g������Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ
���������·�Ӧ2NH3��g��?N2��g��+3H2��g����ƽ�ⳣ��Ϊ ��
��5������N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
��֪��N2��g��+O2��g���T2NO��g����H=+180.5kJ?mol-1
N2��g��+3H2��g���T2NH3��g����H=-92.4kJ?mol-1
2H2��g��+O2��g���T2H2O��g����H=-483.6kJ?mol-1
����������������һ�����������ˮ�������Ȼ�ѧ����ʽΪ ��
��6���Է�ӦN2O4��g��?2NO2��g�������¶ȷֱ�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ2��ʾ������˵����ȷ���� ��
A��A��C����ķ�Ӧ���ʣ�A��C
B��B��C����������ƽ����Է���������B��C
C��A��C�����������ɫ��A�Cdz
D����״̬B��״̬A�������ü��ȵķ���
��7������H2��O2��������Na2CO3��ɵ�ȼ�ϵ�أ����õ�ⷨ�Ʊ�N2O5��װ����ͼ3��ʾ������YΪCO2��д��ʯīI�缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ ���ڵ���������1molN2O5ת�Ƶ��ӵ����ʵ���Ϊ ��
| T/K | 303 | 313 | 323 | 353 |
| NH3������/��10-6 mol�� | 4.8 | 5.9 | 6.0 | 2.0 |
| 3 |
| 2 |
�ش��������⣺
��1���÷�Ӧ�ڽϵ��¶����ܷ��Է����У�
��2����323K��353K�����������������ٵ�ԭ��
��3���뻭��������Ӧ���д�����������������·�Ӧ��������ϵ�����仯ʾ��ͼ1�������б�Ҫ��ע��

��4����ҵ�ϳɰ��ķ�ӦΪN2��g��+3H2��g��?2NH3��g���������ݻ�Ϊ2.0L���ܱ������г���0.60mol N2��g����1.60mol H2��g������Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ
| 4 |
| 7 |
��5������N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
��֪��N2��g��+O2��g���T2NO��g����H=+180.5kJ?mol-1
N2��g��+3H2��g���T2NH3��g����H=-92.4kJ?mol-1
2H2��g��+O2��g���T2H2O��g����H=-483.6kJ?mol-1
����������������һ�����������ˮ�������Ȼ�ѧ����ʽΪ
��6���Է�ӦN2O4��g��?2NO2��g�������¶ȷֱ�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ2��ʾ������˵����ȷ����
A��A��C����ķ�Ӧ���ʣ�A��C
B��B��C����������ƽ����Է���������B��C
C��A��C�����������ɫ��A�Cdz
D����״̬B��״̬A�������ü��ȵķ���
��7������H2��O2��������Na2CO3��ɵ�ȼ�ϵ�أ����õ�ⷨ�Ʊ�N2O5��װ����ͼ3��ʾ������YΪCO2��д��ʯīI�缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ
��֪�ϳɰ���ӦN2��g��+3H2��g��?2NH3��g����H=-92.30kJ?mol-1����ij�¶���2L���ܱ������н��У�����������ݣ�
����˵����ȷ���ǣ�������
| ʱ�䣨h�� ���ʵ�����mol�� |
0 | 1 | 2 | 3 | 4 |
| N2 | 1.50 | n1 | 1.20 | n3 | n5 |
| H2 | 4.50 | 4.20 | 3.60 | n4 | n6 |
| NH3 | O | O��20 | n2 | 1��OO | 1��OO |
| A����Ӧ3h�ڣ���Ӧ������v��N2��ΪO.17 mol?L-1?h-1 |
| B�����¶��£��÷�Ӧ��ƽ�ⳣ��ΪO.037 |
| C����Ӧ���е�1Сʱʱ�ų�������Ϊ9.23 kJ |
| D��4hʱ�����ټ���1 molN2���ﵽ�µĻ�ѧƽ��ʱ��N2��ת������ԭ�������� |
 2NH3��g��+
2NH3��g��+