��Ŀ����
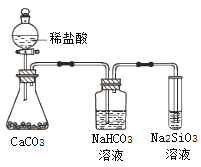
����Ŀ��ijѧϰС��ѧϰ�������ε����ʺ���̽��FeSO4��Һ�ֱ���Na2CO3��Һ��NaHCO3��Һ�ķ�Ӧ����֪��Fe��OH��2��FeCO3��Ϊ��ɫ������������Fe��HCO3��2��ʵ������������¼���£�
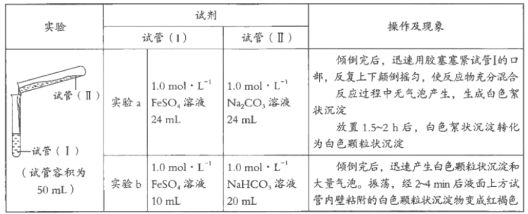
��1����ͬѧ��Ϊʵ��a�а�ɫ����״������FeCO3��д���÷�Ӧ�����ӷ���ʽ��_________________________����Ϊ��֤ʵ�Լ��Ĺ۵㣬����ʵ�飺ȡ������ɫ����״����������__________�����ֲ����������ݡ�
��2����ͬѧ�Ʋ�ʵ��a�İ�ɫ����״�����л����ܺ���Fe��OH��2������ʵ��a��������Һ�������Ϊ15mL���ٽ���ʵ�飬֤ʵ�������Ʋ⡣��֤��Fe��OH��2���ڵ�ʵ��������___________________________��
��3��ʵ��b�а�ɫ����״������Ҫ�ɷ�ҲΪFeCO3��д������FeCO3�����ӷ���ʽ��_____________________________��
��4��ʵ��b��Һ���Ϸ��Թ��ڱ�ճ���İ�ɫ����״�������Ϊ���ɫ����Ҫԭ���dz�ʪ��FeCO3������������д���÷�Ӧ�Ļ�ѧ����ʽ��_________________��
��5����ͬѧ��˼��ʵ��a�к���Fe��OH��2��ʵ��b�м���������Fe��OH��2���Աȷ������ֲ����ԭ����_____________________________��
���𰸡�Fe2++CO32-=FeCO3�� ϡ���ᣨ��ϡ���ᣩ ������ɫ�ɰ�ɫ��Ϊ����ɫ������Ϊ���ɫ���� Fe2++2HCO3-=FeCO3��+CO2��+H2O 4FeCO3+O2+6H2O=4Fe(OH)3+4CO2 ��Ϻ���Ȼʵ��b��̼������Ũ�ȱ�ʵ��a��̼����Ũ�ȸߣ�����̼������Һ�ļ��ԣ���ˮ��̶ȣ���̼�����Ƶ�ǿ
��������
�������ҪץסFeCO3��Fe(OH)2�����������з�����FeCO3�����ᷴӦ�������壻Fe(OH)2�ܱ������е�������������������ɫ�仯���ݴ˷��������
��1��ʵ��a������������̼������Һ���������̼�����������������˸��ֽⷴӦ����Ӧ�����ӷ���ʽΪ��Fe2++CO32-=FeCO3����̼������������������������Ტ����������̼���壬����������������������ᷴӦ����������壬�����ϡ���������������̼�������������������������ʴ�Ϊ��Fe2++CO32-=FeCO3����ϡ���ᣨ��ϡ���ᣩ��
��2���������к���Fe��OH��2�������ڿ����лᱻ���������ֳ���ɫ����Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ�����ʴ�Ϊ��������ɫ�ɰ�ɫ��Ϊ����ɫ������Ϊ���ɫ������
��3��ʵ��b�еķ�Ӧ��Ϊ����������̼�����ƣ����߷�Ӧ��������̼���������������ж�����̼�����ˮ���ɣ���Ӧ�����ӷ���ʽΪ��Fe2++2HCO3-=FeCO3��+CO2��+H2O���ʴ�Ϊ��Fe2++2HCO3-=FeCO3��+CO2��+H2O��
��4��FeCO3�������������ɺ��ɫ��������������Ӧ������Ϊ��������̼������Ϊ��ԭ������������ԭ��Ӧ���ɿɵ÷�Ӧ��4FeCO3+O2+6H2O=4Fe(OH)3+4CO2���ʴ�Ϊ��4FeCO3+O2+6H2O=4Fe(OH)3+4CO2��
��5���Ա�ʵ��a��b����֮ͬ������ǰ�߷�Ӧ��Ϊ̼������Һ������Ϊ̼��������Һ������̼������ӵ�ˮ��̶Ƚ�̼��������ӵ�ˮ���ʵ��a�л����Һ����������Ũ�Ƚ�ʵ��b��Ҫ����ʵ��a�и��ײ���Fe��OH��2���ʴ�Ϊ����Ϻ���Ȼʵ��b��̼������Ũ�ȱ�ʵ��a��̼����Ũ�ȸߣ�����̼������Һ�ļ��ԣ���ˮ��̶ȣ���̼�����Ƶ�ǿ��

 ��У����ϵ�д�
��У����ϵ�д�