��Ŀ����
���������������������ˮ��ҵ��ˮ����������������������Ͽ졣ʵ���ҿ��ö�������Ϊ��Ҫԭ���Ʊ�������ء��䲿���������£�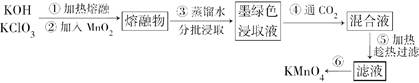
(1)�ڢٲ��в��������������ô�������ԭ����(�û�ѧ����ʽ��ʾ)________________________________________��
(2)KOH��KClO3��MnO2���۷�Ӧ����ī��ɫK2MnO4�Ļ�ѧ����ʽΪ________________________________________��
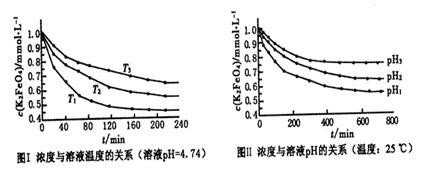
(3)�ڢܲ�ͨ��CO2����ʹMnO42��������Ӧ������MnO4����MnO2����K2MnO4��ȫ��Ӧʱ��ת��ΪKMnO4�İٷ���ԼΪ________(��ȷ��0.1%)��
(4)�ڢݲ����ȹ��˵�Ŀ����______________________________________��
(5)�ڢ�����Ũ����Һ����ϸС��������ʱ��ֹͣ���ȣ���ȴ�ᾧ��________��ϴ�ӡ������������У��¶Ȳ��˹��ߣ���Ϊ____________________________________��
(1)SiO2��2KOH=K2SiO3��H2O
(2)6KOH��KClO3��3MnO2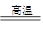 KCl��3K2MnO4��3H2O
KCl��3K2MnO4��3H2O
(3)66.7 %
(4)���ٹ���ʱ�����(���ֹ���¹�����KMnO4���������)
(5)���ˡ��¶ȹ���KMnO4��ֽ�
����

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д����÷Ͼɶ�п��Ƥ���Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ�����ͼ���£�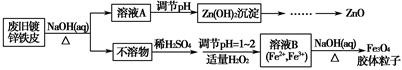
��֪��Zn���仯�����������Al���仯������������ơ���ش��������⣺
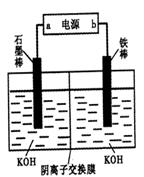
��1����NaOH��Һ�����Ͼɶ�п��Ƥ��������________��
| A��ȥ������ | B���ܽ��п�� | C��ȥ������ | D���ۻ� |
��3������ҺB�Ƶ�Fe3O4�������ӵĹ����У������ͨ��N2����ԭ����_________________________________________________________��
��4�����ظ���ط���һ��������ԭ�ζ������ɲⶨ����Fe3O4�еĶ�������������������Ũ��Ϊ0.010 00 mol��L��1��K2Cr2O7����Һ250 mL��Ӧȷ��ȡ________g K2Cr2O7������4λ��Ч���֣���֪MK2Cr2O7��294.0 g��mol��1�������Ƹñ���Һʱ�����������в���Ҫ�õ�����________���ñ�ű�ʾ����
�ٵ�����ƽ�����ձ�������Ͳ���ܲ�������������ƿ����ͷ�ιܣ�����Һ��
��5���ζ������У�����ζ�ǰװ��K2Cr2O7����Һ�ĵζ��ܼ��첿�������ݣ����ζ�������������ʧ����ⶨ�����________���ƫ��ƫС�����䡱����
Fe2����I�������ֳ����Ļ�ԭ�����ӡ�
��1����FeSO4��Һ�еμ���ˮ����Һ��dz��ɫ��ɻ�ɫ����Ӧ�����ӷ���ʽΪ________________________����KI��Һ�еμ���ˮ����Һ����ɫ��ɻ�ɫ����Ӧ�����ӷ���ʽΪ______________________��
��2������FeSO4��Һ��KI��Һ����ˮ��2% KSCNΪ�Լ�֤��I���Ļ�ԭ��ǿ��Fe2�������ʵ�鷽�����������ʵ�鲽�衢Ԥ������ͽ��ۡ�
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ2mLFeSO4��Һ��2mLKI��Һ������Թ��У��ٵμ�1~2����ˮ�� | ������Һ��ɻ�ɫ�� ���ۣ� �� |
| ����2��__________________________ __________________________________ | ���� �� ���ۣ� |
��3������ ���ṩ���Լ�֤���������Ļ����������ԣ�2�ۣ�ʵ������������ǣ�ȡ������Ʒ����ˮ�� ��
��4����2mol FeI2��Һ��ͨ��2.5mol Cl2ʱ����д���ܵ����ӷ���ʽ�� ��
 (NxOy)�� H2O(��ƽʱx��y�þ�����ֵ��ʾ����������
(NxOy)�� H2O(��ƽʱx��y�þ�����ֵ��ʾ���������� TiO2��H2O
TiO2��H2O 3Cu2++2R+yH2O��
3Cu2++2R+yH2O�� Fe(OH)3��5OH��]
Fe(OH)3��5OH��]

 �÷�Ӧ�У�____________Ԫ�ر���ԭ���÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ__________��
�÷�Ӧ�У�____________Ԫ�ر���ԭ���÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ__________��
 _____Cu+_____CuCl2+N2��+_____H2O��
_____Cu+_____CuCl2+N2��+_____H2O��