��Ŀ����
��14�֣��״�����Ϊ21���͵�����ȼ�ϣ���ҵ��ͨ�����з�Ӧ�ٺ͢ڣ���CH4��H2OΪԭ�����Ʊ��״�����CH4��g����H2O ��g�� CO��g����3H2��g�� ��H1 ��CO��g����2H2��g��
CO��g����3H2��g�� ��H1 ��CO��g����2H2��g�� CH3OH��g����H2 ��0��2mol CH4��0��3mol H2O��g��ͨ���ݻ�Ϊ10L���ܱ������У���һ�������·�����Ӧ�٣��ﵽƽ��ʱ��CH4��ת�������¶ȣ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH��g����H2 ��0��2mol CH4��0��3mol H2O��g��ͨ���ݻ�Ϊ10L���ܱ������У���һ�������·�����Ӧ�٣��ﵽƽ��ʱ��CH4��ת�������¶ȣ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
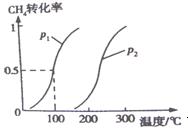
��1���¶Ȳ��䣬��С���������ѹǿ���ٵķ�Ӧ���� �����������С���������䡱����ƽ���� �����ƶ���
��2����Ӧ�ٵġ�H1 0���������������������������ƽ�ⳣ������ ʽΪK= ��100�棬ѹǿΪp1ʱƽ�ⳣ����ֵ�� ��
ʽΪK= ��100�棬ѹǿΪp1ʱƽ�ⳣ����ֵ�� ��
��3����ѹǿΪ0��1Mpa�����£���a mol CO��3a molH2�Ļ�������ڴ��������½��з�Ӧ�ڡ�Ϊ��Ѱ�Һϳɼ״����¶Ⱥ�ѹǿ������������ijͬѧ���������ʵ�飬����ʵ�������Ѿ���������ʵ����Ʊ��С������¿ո�������ʣ���ʵ���������ݡ�
��4�������꿪���ļ״�ȼ�ϵ���Dz��ò��缫������е����ӽ���Ĥֻ�������Ӻ�ˮ����ͨ�����乤��ԭ��ʾ��ͼ���£�

��ش�
��Pt��a���缫�ĵ缫��ӦʽΪ
������õ�ع���ʱ��·��ͨ��2mol���������ĵ�CH3OH�� mol��
 CO��g����3H2��g�� ��H1 ��CO��g����2H2��g��
CO��g����3H2��g�� ��H1 ��CO��g����2H2��g�� CH3OH��g����H2 ��0��2mol CH4��0��3mol H2O��g��ͨ���ݻ�Ϊ10L���ܱ������У���һ�������·�����Ӧ�٣��ﵽƽ��ʱ��CH4��ת�������¶ȣ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH��g����H2 ��0��2mol CH4��0��3mol H2O��g��ͨ���ݻ�Ϊ10L���ܱ������У���һ�������·�����Ӧ�٣��ﵽƽ��ʱ��CH4��ת�������¶ȣ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
��1���¶Ȳ��䣬��С���������ѹǿ���ٵķ�Ӧ���� �����������С���������䡱����ƽ���� �����ƶ���
��2����Ӧ�ٵġ�H1 0���������������������������ƽ�ⳣ������
 ʽΪK= ��100�棬ѹǿΪp1ʱƽ�ⳣ����ֵ�� ��
ʽΪK= ��100�棬ѹǿΪp1ʱƽ�ⳣ����ֵ�� ����3����ѹǿΪ0��1Mpa�����£���a mol CO��3a molH2�Ļ�������ڴ��������½��з�Ӧ�ڡ�Ϊ��Ѱ�Һϳɼ״����¶Ⱥ�ѹǿ������������ijͬѧ���������ʵ�飬����ʵ�������Ѿ���������ʵ����Ʊ��С������¿ո�������ʣ���ʵ���������ݡ�
| ʵ���� | T�� | n��CO��/n��H2�� | p ��Mpa�� |
| I | 150 | 1/3 | 0��1 |
| �� | | | 5 |
| �� | 350 | | 5 |

��ش�
��Pt��a���缫�ĵ缫��ӦʽΪ
������õ�ع���ʱ��·��ͨ��2mol���������ĵ�CH3OH�� mol��
��1������1�֣� �淴Ӧ��������1�֣�
��2������1�֣� c ��CO����c3��H2��/c ��CH4����c��H2O����2�֣� 1��35��10-3��2�֣�
��3��II 150 1/3 III 1/3 ��ÿ��1�ֹ�3�֣�
��4����CH3OH + H2O -6e- = CO2��+ 6 H+ ��2�֣�
��1/3 ��2�֣�
��2������1�֣� c ��CO����c3��H2��/c ��CH4����c��H2O����2�֣� 1��35��10-3��2�֣�
��3��II 150 1/3 III 1/3 ��ÿ��1�ֹ�3�֣�
��4����CH3OH + H2O -6e- = CO2��+ 6 H+ ��2�֣�
��1/3 ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 2NH3������˵���������
2NH3������˵��������� 2NO2����H ��0����ÿ��һ��ʱ��������ڵ����ʽ��вⶨ���õ��������ݣ�
2NO2����H ��0����ÿ��һ��ʱ��������ڵ����ʽ��вⶨ���õ��������ݣ� ���N2O4��ת���ʽ� �����������С�����䡱����
���N2O4��ת���ʽ� �����������С�����䡱���� CH3OH(g)
CH3OH(g)
 ������ձ��У�������ˮϡ����500mL����ʱx�����Ỻ�͵ؽ��з�Ӧ�����з�Ӧ����������
������ձ��У�������ˮϡ����500mL����ʱx�����Ỻ�͵ؽ��з�Ӧ�����з�Ӧ����������



 3C(g)��4D(g)�У���������ܹ����÷�Ӧ���ʵ���
3C(g)��4D(g)�У���������ܹ����÷�Ӧ���ʵ��� H2(g)��I2(g)�ﵽƽ�⣬����˵���п϶���ȷ���ǣ� ��
H2(g)��I2(g)�ﵽƽ�⣬����˵���п϶���ȷ���ǣ� ��