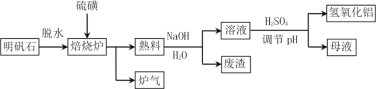
��Ŀ����
����Ŀ��A��B��C��D��E��F����Ԫ�ص�ԭ���������ε�������֪����F��ԭ������Ϊ29������ľ�Ϊ����������Ԫ�أ���Eԭ�Ӽ۵���(��Χ����)�Ų�Ϊmsnmpn��1����Dԭ������������Ϊż������A��Cԭ��p����ĵ������ֱ�Ϊ2��4��
��ش��������⣺
(1)����������ȷ����________(�����)��
A����������ǿ����D��E
B����̬ԭ�ӵ�һ�����ܣ�D��E
C������Ԫ���У��縺������Ԫ����E
D�������ܣ�NaCl��DCl2
(2)F��̬ԭ�ӵĺ�������Ų�ʽΪ_____����Fͬһ���ڵĸ���Ԫ�صĻ�̬ԭ����������������Fԭ����ͬ��Ԫ��Ϊ_____(��Ԫ�ط���)��
(3)E���ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��

����֪E��ԭ�Ӱ뾶Ϊd��NA���������ӵ�������E�����ԭ������ΪMr����һ��������Eԭ�ӵ���ĿΪ________���þ�����ܶ�Ϊ_______________ (����ĸ��ʾ)��
���𰸡�BD 1s22s22p63s23p63d104s1 Cr 4 Mr/(4��21/2 d3NA)
��������
A��B��C��D��E��F����Ԫ�ص�ԭ���������ε�������FԪ���⣬����ľ�Ϊ����������Ԫ�ء���F��ԭ������Ϊ29����FΪͭԪ�أ���A��Cԭ��p����ĵ������ֱ�Ϊ2��4����A��������Ų�ʽΪ1s22s22p2����AΪ̼Ԫ�أ�C��������Ų�ʽΪ1s22s22p4����CΪ��Ԫ�أ�BԪ��ԭ����������̼Ԫ������Ԫ��֮�䣬��BΪ��Ԫ�أ���Eԭ�Ӽ۵���(��Χ����)�Ų�Ϊmsnmpn-1��s�ܼ�����2�����ӣ���E��Χ�����Ų�Ϊms2mp1��ԭ������������Ԫ�أ���EΪ��Ԫ�أ���Dԭ������������Ϊż����Dԭ������������Ԫ������Ԫ��֮�䣬��DΪþԪ�ء��ݴ˽��Ԫ�������ɺ;��������֪ʶ�������
��������������AΪ̼Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�DΪþԪ�أ�EΪ��Ԫ�أ�FΪͭԪ�ء�
(1)A��DΪþԪ�أ�EΪ��Ԫ�أ������Ӱ뾶С��þ���Ӱ뾶�������ļ۵�����Ŀ����þ�����Խ�����D��E����A����B��ͬһ�����У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�������A�塢��VA��Ԫ�ص�һ�����ܴ�������Ԫ�أ�����D��E����B��ȷ��C���ǽ�����Խǿ���縺��Խ������Ԫ���У��縺������Ԫ����O(C)����C����D�����Ӱ뾶�����Ӵ���þ���ӣ��������ӵ����С��þ���ӣ��Ȼ�þ�����Ӽ���ǿ�������ܣ�NaCl��MgCl2����D��ȷ����ѡBD��
(2)FΪͭԪ�أ�����������Ϊ29�����������Ϊ29����������Ų�ʽ��1s22s22p63s23p63d104s1����Fͬһ���ڵĸ���Ԫ�صĻ�̬ԭ����������������Fԭ����ͬ��Ԫ�أ��۲�����Ų�Ϊ3d54s1����������Ų�ʽ��1s22s22p63s23p63d54s1����CrԪ�أ��ʴ�Ϊ��1s22s22p63s23p63d104s1��Cr��
(3)������Alԭ����ĿΪ��8��![]() +6��
+6��![]() =4���þ���������m=4��
=4���þ���������m=4��![]() �����ݱ�ͼ��֪����������Խ���Ϊ4d�������ⳤ=
�����ݱ�ͼ��֪����������Խ���Ϊ4d�������ⳤ=![]() ��4d�����������V=(4��d��
��4d�����������V=(4��d��![]() )3=16
)3=16![]() d3�������ܶ���=
d3�������ܶ���=![]() =
=![]() ���ʴ�Ϊ��4��
���ʴ�Ϊ��4��![]() ��
��

����Ŀ���±������ۺ�������( )
ѡ�� | ��ѧ��Ӧ�������ӷ���ʽ | ���� |
A | ����ϡ���ᷴӦ�� | ��ȷ |
B | ����ʯ���ڴ���ķ�Ӧ�� | ������ӦдΪ������ʽCH3COOH��CaCO3Ӧд��������ʽ |
C | ��Ba(OH)2��Һ�еμ�����NaHCO3��Һ��Ba2++ OH-+HCO3- = BaCO3��+ H2O | ������ʽ��Ba2+��OH-��ѧ������֮��Ϊ1��2 |
D | NH4HCO3��Һ�����KOHŨ��Һ���ȣ� | ����HCO3-Ҳ������OH����Ӧ |
A.AB.BC.CD.D
����Ŀ����ͬԪ�ص�ԭ���ڷ������������ӵ�������С����һ����ֵx����ʾ����xԽ����ԭ���������ӵ�����Խǿ�������γɵķ����г�Ϊ�����һ����
������ijЩ������Ԫ�ص�xֵ��
Ԫ�ط��� | Li | Be | B | C | O | F | Na | Al | Si | P | S | Cl |
xֵ | 0.98 | 1.57 | 2.04 | 2.55 | 3.44 | 3.98 | 0.93 | 1.61 | 1.90 | 2.19 | 2.58 | 3.16 |
��1��ͨ������xֵ�仯���ɣ�ȷ��Mg��xֵ��Χ��____<x(Mg)< _____��
��2���Ʋ�xֵ��ԭ�Ӱ뾶�Ĺ�ϵ��____�����ݶ�����Ԫ�ص�xֵ�仯�ص㣬������Ԫ�����ʵ�________�仯���ɡ�
��3��ij�л�������ṹʽΪ![]() ������S��N�У�����Ϊ���õ��Ӷ�ƫ��˭��__��дԭ�����ƣ���
������S��N�У�����Ϊ���õ��Ӷ�ƫ��˭��__��дԭ�����ƣ���
��4��������ɸ������ǣ����ɼ�����ԭ����ӦԪ�صIJ�ֵ����x��������x>1.7ʱ��һ��Ϊ���Ӽ�������x<1.7ʱ��һ��Ϊ���ۼ������ƶ�AlBr3�л�ѧ��������______��
��5��Ԥ��Ԫ�����ڱ��У�xֵ��С��Ԫ��λ�ã�_________