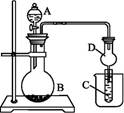
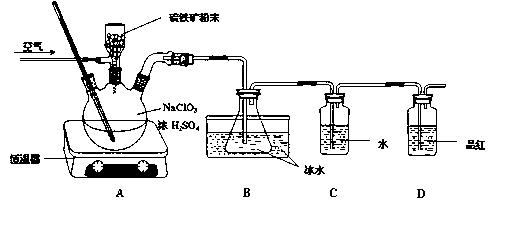
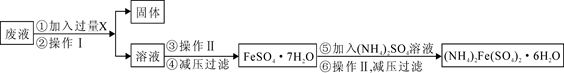
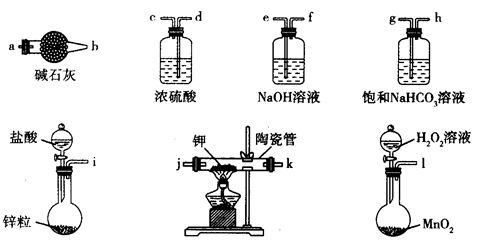
��Ŀ����
�������ƾ���[CaO2��8H2O]���ȶ����ʰ�ɫ������ˮ��������������Һ���㷺Ӧ���ڻ���ɱ��������������
��.�������ƾ�����Ʊ���
��ҵ������CaO2��8H2O����Ҫ�������£�
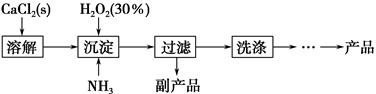
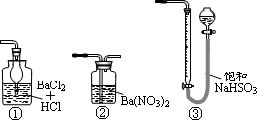
��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ��_________________________________��
��2������ʱ���ñ�ˮ�����¶���10 �����º�ͨ�������NH3�������ԭ��ֱ��Ǣ�__________________________����_____________________________��
��.�������ƾ��庬���IJⶨ��
ȷ��ȡ0.300 0 g��Ʒ����ƿ�У�����30 mL����ˮ��10 mL 2.000 mol��L��1 H2SO4����0.020 0 mol��L��1 KMnO4����Һ�ζ����յ㡣�ظ������������Ρ�H2O2��KMnO4��Ӧ�����ӷ���ʽΪ2MnO4-��5H2O2��6H��=2Mn2����5O2����8H2O
�ζ��յ�۲쵽������Ϊ_______________________________________��
��4�����ݱ������ݣ������Ʒ��CaO2��8H2O������������д��������̣���
KMnO4����Һ�ζ�����
��.�������ƾ�����Ʊ���
��ҵ������CaO2��8H2O����Ҫ�������£�

��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ��_________________________________��
��2������ʱ���ñ�ˮ�����¶���10 �����º�ͨ�������NH3�������ԭ��ֱ��Ǣ�__________________________����_____________________________��
��.�������ƾ��庬���IJⶨ��
ȷ��ȡ0.300 0 g��Ʒ����ƿ�У�����30 mL����ˮ��10 mL 2.000 mol��L��1 H2SO4����0.020 0 mol��L��1 KMnO4����Һ�ζ����յ㡣�ظ������������Ρ�H2O2��KMnO4��Ӧ�����ӷ���ʽΪ2MnO4-��5H2O2��6H��=2Mn2����5O2����8H2O
�ζ��յ�۲쵽������Ϊ_______________________________________��
��4�����ݱ������ݣ������Ʒ��CaO2��8H2O������������д��������̣���
KMnO4����Һ�ζ�����
| �ζ����� | ��Ʒ������/g | KMnO4��Һ�����/mL | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 0.300 0 | 1.02 | 24.04 |
| 2 | 0.300 0 | 2.00 | 25.03 |
| 3 | 0.300 0 | 0.20 | 23.24 |
��.��1��CaCl2��H2O2��2NH3��8H2O=CaO2��8H2O����2NH4Cl����2�����¶ȵͿɼ��ٹ�������ķֽ⣬��߹�������������ʣ����ֹ��������ķֽ⣩����ͨ�����NH3ʹ��Һ�ʼ��ԣ�����CaO2��8H2O���ܽ⣨��ʹ��Һ�ʼ��ԣ�����CaO2��8H2O���ܽ⣬����߲�Ʒ�IJ��ʣ�
��.��3�����������һ��KMnO4����Һ����Һ����ɫ��Ϊdz�Ϻ�ɫ����30 s����ɫ
��4�����ݷ�Ӧ����ʽ2MnO4-��5H2O2��6H��=2Mn2����5O2����8H2O��CaCl2��H2O2��2NH3��8H2O=CaO2��8H2O����2NH4Cl����������ʽ��
5��CaO2��8H2O����5H2O2��2KMnO4
n��CaO2��8H2O����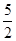 n��KMnO4����
n��KMnO4����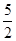 ��0.020 0 mol��L��1��23.03 mL��10��3 L��mL��1��1.151 5��10��3 mol
��0.020 0 mol��L��1��23.03 mL��10��3 L��mL��1��1.151 5��10��3 mol
CaO2��8H2O������������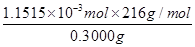 ��100%��82.91%��
��100%��82.91%��
��.��3�����������һ��KMnO4����Һ����Һ����ɫ��Ϊdz�Ϻ�ɫ����30 s����ɫ
��4�����ݷ�Ӧ����ʽ2MnO4-��5H2O2��6H��=2Mn2����5O2����8H2O��CaCl2��H2O2��2NH3��8H2O=CaO2��8H2O����2NH4Cl����������ʽ��
5��CaO2��8H2O����5H2O2��2KMnO4
n��CaO2��8H2O����
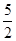 n��KMnO4����
n��KMnO4����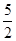 ��0.020 0 mol��L��1��23.03 mL��10��3 L��mL��1��1.151 5��10��3 mol
��0.020 0 mol��L��1��23.03 mL��10��3 L��mL��1��1.151 5��10��3 molCaO2��8H2O������������
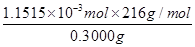 ��100%��82.91%��
��100%��82.91%����1����������ͼ�еļ�ͷָ��ȷ����Ӧ����CaCl2��H2O2��NH3��������CaO2��8H2O�����÷�Ӧǰ��Ԫ���غ㣬ȷ����һ��ӦΪH2O��������ΪNH4Cl���ʷ�Ӧ�Ļ�ѧ����ʽΪCaCl2��H2O2��2NH3��8H2O=CaO2��8H2O����2NH4Cl����2��30%H2O2�����ֽ⣬��˳���ʱ���ñ�ˮ�����¶���10 �����¼��ٹ�������ķֽ⣬��߹�������������ʣ�����ͨ��NH3�IJ���Ϊ��������������ͨ�����NH3ʹ��Һ�ʼ��ԣ�����CaO2��8H2O���ܽ⣬��ʹ���������3��������0.020 0 mol��L��1 KMnO4����Һ����ɫ�����еζ������Եζ��յ�۲쵽������Ϊ���������һ��KMnO4����Һ����Һ����ɫ��Ϊdz�Ϻ�ɫ����30 s�ڲ���ɫ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 ������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00ml����ش��������⣺
������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00ml����ش��������⣺