��Ŀ����
��������;�㷺����Ҫ��������Ӳ�ʻ����µĺϽ��Լ����ݵĵ�˿�������£����ܱ���������H2��ԭWO3�ɵõ������٣����ܷ�ӦΪ��
WO3 (s) + 3H2 (g) W (s) + 3H2O (g)
W (s) + 3H2O (g)
��ش��������⣺
��������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ___________________________��
��ij�¶��·�Ӧ��ƽ��ʱ��H2��ˮ�����������Ϊ2:3����H2��ƽ��ת����Ϊ_____________________�����¶ȵ����ߣ�H2��ˮ����������ȼ�С����÷�ӦΪ��Ӧ_____________________������ȡ����ȡ�����
�������ܷ�Ӧ���̴��·�Ϊ�����Σ�������Ҫ�ɷ����¶ȵĹ�ϵ���±���ʾ��
��һ�η�Ӧ�Ļ�ѧ����ʽΪ___________________________��580��ʱ���������ʵ���Ҫ�ɷ�Ϊ________������WO3��ȫת��ΪW��������������H2���ʵ���֮��Ϊ____________________________________��
�� ��֪���¶ȹ���ʱ��WO2 (s)ת��ΪWO2 (g)��
WO2 (s) + 2H2 (g) W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1
W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1
WO2 (g) + 2H2(g) W (s) + 2H2O (g)����H �� ��137.9 kJ��mol��1
W (s) + 2H2O (g)����H �� ��137.9 kJ��mol��1
��WO2 (s) WO2 (g) �Ħ�H �� ______________________��
WO2 (g) �Ħ�H �� ______________________��
����˿�ƹ��е�W��ʹ�ù����л����ӷ���ʹ��˿��ϸ������I2���ӳ��ƹܵ�ʹ���������乤��ԭ��Ϊ��W (s) +2I2 (g) WI4 (g)������˵����ȷ����____________��
WI4 (g)������˵����ȷ����____________��
a���ƹ��ڵ�I2��ѭ��ʹ��
b��WI4�ڵ�˿�Ϸֽ⣬������W�ֳ����ڵ�˿��
c��WI4�ڵƹܱ��Ϸֽ⣬ʹ�ƹܵ������ӳ�
d���¶�����ʱ��WI4�ķֽ����ʼӿ죬W��I2�Ļ������ʼ���
WO3 (s) + 3H2 (g)
 W (s) + 3H2O (g)
W (s) + 3H2O (g) ��ش��������⣺
��������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ___________________________��
��ij�¶��·�Ӧ��ƽ��ʱ��H2��ˮ�����������Ϊ2:3����H2��ƽ��ת����Ϊ_____________________�����¶ȵ����ߣ�H2��ˮ����������ȼ�С����÷�ӦΪ��Ӧ_____________________������ȡ����ȡ�����
�������ܷ�Ӧ���̴��·�Ϊ�����Σ�������Ҫ�ɷ����¶ȵĹ�ϵ���±���ʾ��
| �¶� | 25�� ~ 550�� ~ 600�� ~ 700�� |
| ��Ҫ�ɷ� | WO3 W2O5 WO2 W |
��һ�η�Ӧ�Ļ�ѧ����ʽΪ___________________________��580��ʱ���������ʵ���Ҫ�ɷ�Ϊ________������WO3��ȫת��ΪW��������������H2���ʵ���֮��Ϊ____________________________________��
�� ��֪���¶ȹ���ʱ��WO2 (s)ת��ΪWO2 (g)��
WO2 (s) + 2H2 (g)
 W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1
W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1 WO2 (g) + 2H2(g)
 W (s) + 2H2O (g)����H �� ��137.9 kJ��mol��1
W (s) + 2H2O (g)����H �� ��137.9 kJ��mol��1 ��WO2 (s)
 WO2 (g) �Ħ�H �� ______________________��
WO2 (g) �Ħ�H �� ______________________������˿�ƹ��е�W��ʹ�ù����л����ӷ���ʹ��˿��ϸ������I2���ӳ��ƹܵ�ʹ���������乤��ԭ��Ϊ��W (s) +2I2 (g)
 WI4 (g)������˵����ȷ����____________��
WI4 (g)������˵����ȷ����____________��a���ƹ��ڵ�I2��ѭ��ʹ��
b��WI4�ڵ�˿�Ϸֽ⣬������W�ֳ����ڵ�˿��
c��WI4�ڵƹܱ��Ϸֽ⣬ʹ�ƹܵ������ӳ�
d���¶�����ʱ��WI4�ķֽ����ʼӿ죬W��I2�Ļ������ʼ���
��1��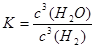 ��2��60% ����
��2��60% ����
��3��2WO3+H2=W2O5+H2O�� W2O5��WO2�� 1:1:4��
��4��+203.9KJ.mol-1 ��5�� a��b��
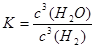 ��2��60% ����
��2��60% ������3��2WO3+H2=W2O5+H2O�� W2O5��WO2�� 1:1:4��
��4��+203.9KJ.mol-1 ��5�� a��b��
���������
��1�����ݷ���ʽ�ɵá�
��2��H2��ˮ����������ȼ�С��˵����Ӧ�����ƶ���������Ӧ�����ȷ�Ӧ��
��3���ɱ�����Ϣ��֪����һ�η�Ӧʱ��2WO3+H2=W2O5+H2O����Ҫ�ɷ�Ϊ��W2O5��WO2��
��4�����ݸ�˹���ɣ���H ��+203.9 KJ.mol-1
��5��a���ⵥ���������������ظ�ʹ�á�
b��WI4�ڵ�˿�Ϸֽ��������³�����

��ϰ��ϵ�д�
�����Ŀ
 2Cl2(g)+2H2O(g) ��H=" ��115.6" kJ/mol
2Cl2(g)+2H2O(g) ��H=" ��115.6" kJ/mol 

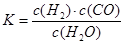 ��������Ӧ�Ļ�ѧ����ʽΪ��
��������Ӧ�Ļ�ѧ����ʽΪ��  2NH3(g) ��H<0 ��K=0.5����400��ʱ����0.5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��Ӧv(N2)�� v(N2)���������������������ȷ������1�֣�
2NH3(g) ��H<0 ��K=0.5����400��ʱ����0.5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��Ӧv(N2)�� v(N2)���������������������ȷ������1�֣�
 2SO3(g) ��H����196.6 kJ��mol��1��Ӧ���̵������仯ʾ��ͼ��
2SO3(g) ��H����196.6 kJ��mol��1��Ӧ���̵������仯ʾ��ͼ��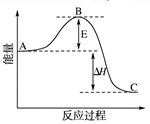
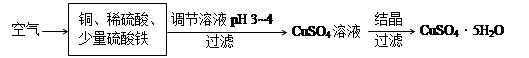
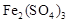 ������ã�ʹ��Ӧ������������ɣ�
������ã�ʹ��Ӧ������������ɣ�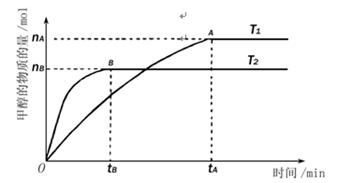
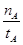 (mol��L-1��min-1)
(mol��L-1��min-1)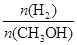 ��С
��С 2NH3��g������Ӧ���̵������仯��ͼ��ʾ����֪N2��g����H2��g����Ӧ����17 g NH3��g�����ų�46.1 kJ��������
2NH3��g������Ӧ���̵������仯��ͼ��ʾ����֪N2��g����H2��g����Ӧ����17 g NH3��g�����ų�46.1 kJ��������



 CO(NH2)2(l)+H2O(l)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
CO(NH2)2(l)+H2O(l)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£� ?2NH3(g) ��H2����92.4 kJ��mol��1
?2NH3(g) ��H2����92.4 kJ��mol��1 H2O(l)+H2NCONH2(l) ��H=-103��7 kJ��mol-1
H2O(l)+H2NCONH2(l) ��H=-103��7 kJ��mol-1

 ��
�� ����ԭ
����ԭ �ķ���Ҳ�������������������Ⱦ�����磺
�ķ���Ҳ�������������������Ⱦ�����磺