��Ŀ����
���ڴ�������Ϊ��ѧ��ҵ����������ľ���Ч�棬�����о���Ѱ��һֱ���ܵ����ӵĸ߿Ƽ�����
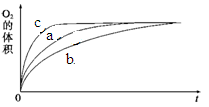
��1��V2O5�ǽӴ���������Ĵ�������ͼΪ��������������2SO2 (g) + O2(g) 2SO3(g) ��H����196.6 kJ��mol��1��Ӧ���̵������仯ʾ��ͼ��
2SO3(g) ��H����196.6 kJ��mol��1��Ӧ���̵������仯ʾ��ͼ��
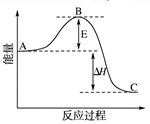
��V2O5��ʹ�û�ʹͼ��B�� ������ߡ��������͡�����
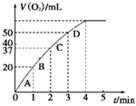
��һ�������£�SO2�������Ӧtmin��SO2��SO3���ʵ���Ũ�ȷֱ�Ϊa mol/L��b mol/L����SO2��ʼ���ʵ���Ũ��Ϊ mol/L������SO3�Ļ�ѧ��Ӧ����Ϊ mol/(L��min)��
��2����ͼ��һ����ͭ��ϡ����Ϊԭ��������������������ʾ��ͼ��
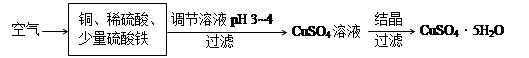
������CuSO4���ܷ�ӦΪ2Cu+O2+2H2SO4��2 CuSO4+2H2O������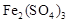 ������ã�ʹ��Ӧ������������ɣ�
������ã�ʹ��Ӧ������������ɣ�
��һ����Cu��2Fe3����2Fe2����Cu2��
�ڶ����� ���������ӷ���ʽ��ʾ��
�ڵ�����ҺpHΪ3��4��Ŀ���� ������ʱ������Լ�����Ϊ ����ѡ����ţ�
a��NaOH��Һ b��CuO��ĩ c��Cu2(OH)2CO3 d����ˮ
��3������TiO2�������Ĺ�����������ҵ�����������Ƶô�TiO2����ת��ΪTiCl4(l)����TiCl4(l)��ȡ����TiO2�ķ���֮һ�ǽ�TiCl4���嵼�����������У�700��1000�棩����ˮ�⡣
��֪��TiO2(s)��2Cl2(g)��TiCl4(l)��O2(g) ��H����140 kJ��mol��1
2C(s)��O2(g)��2CO(g) ��H����221 kJ��mol��1
��д��TiO2�ͽ�̿��������Ӧ����TiCl4��CO���Ȼ�ѧ����ʽ�� ��
��д������TiCl4(l)��ȡ����TiO2�Ļ�ѧ����ʽ�� ��
��1��V2O5�ǽӴ���������Ĵ�������ͼΪ��������������2SO2 (g) + O2(g)
 2SO3(g) ��H����196.6 kJ��mol��1��Ӧ���̵������仯ʾ��ͼ��
2SO3(g) ��H����196.6 kJ��mol��1��Ӧ���̵������仯ʾ��ͼ��
��V2O5��ʹ�û�ʹͼ��B�� ������ߡ��������͡�����
��һ�������£�SO2�������Ӧtmin��SO2��SO3���ʵ���Ũ�ȷֱ�Ϊa mol/L��b mol/L����SO2��ʼ���ʵ���Ũ��Ϊ mol/L������SO3�Ļ�ѧ��Ӧ����Ϊ mol/(L��min)��
��2����ͼ��һ����ͭ��ϡ����Ϊԭ��������������������ʾ��ͼ��
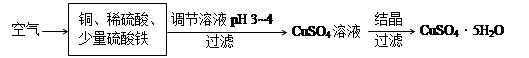
������CuSO4���ܷ�ӦΪ2Cu+O2+2H2SO4��2 CuSO4+2H2O������
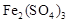 ������ã�ʹ��Ӧ������������ɣ�
������ã�ʹ��Ӧ������������ɣ���һ����Cu��2Fe3����2Fe2����Cu2��
�ڶ����� ���������ӷ���ʽ��ʾ��
�ڵ�����ҺpHΪ3��4��Ŀ���� ������ʱ������Լ�����Ϊ ����ѡ����ţ�
a��NaOH��Һ b��CuO��ĩ c��Cu2(OH)2CO3 d����ˮ
��3������TiO2�������Ĺ�����������ҵ�����������Ƶô�TiO2����ת��ΪTiCl4(l)����TiCl4(l)��ȡ����TiO2�ķ���֮һ�ǽ�TiCl4���嵼�����������У�700��1000�棩����ˮ�⡣
��֪��TiO2(s)��2Cl2(g)��TiCl4(l)��O2(g) ��H����140 kJ��mol��1
2C(s)��O2(g)��2CO(g) ��H����221 kJ��mol��1
��д��TiO2�ͽ�̿��������Ӧ����TiCl4��CO���Ȼ�ѧ����ʽ�� ��
��д������TiCl4(l)��ȡ����TiO2�Ļ�ѧ����ʽ�� ��
��1���ٽ���(1��) ��a+b(1��) 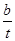 (1��) ��2�� ��4Fe2����O2��4H����4Fe3����2H2O (2��)
(1��) ��2�� ��4Fe2����O2��4H����4Fe3����2H2O (2��)
�ڽ�Fe3+ת��ΪFe(OH)3������ȥ(1��) b,c(2��)
��3����TiO2(s)��2Cl2(g)��2C(s)��TiCl4(l)��2CO(g) ��H����81 kJ��mol��1(2��)
��TiCl4+2H2O TiO2+4HCl(2��)
TiO2+4HCl(2��)
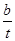 (1��) ��2�� ��4Fe2����O2��4H����4Fe3����2H2O (2��)
(1��) ��2�� ��4Fe2����O2��4H����4Fe3����2H2O (2��) �ڽ�Fe3+ת��ΪFe(OH)3������ȥ(1��) b,c(2��)
��3����TiO2(s)��2Cl2(g)��2C(s)��TiCl4(l)��2CO(g) ��H����81 kJ��mol��1(2��)
��TiCl4+2H2O
 TiO2+4HCl(2��)
TiO2+4HCl(2��)�����������1���ٴ����ܽ��ͷ�Ӧ�Ļ�ܣ�����V2O5��ʹ�û�ʹͼ��B�㽵�͡�
��һ�������£�SO2�������Ӧtmin��SO2��SO3���ʵ���Ũ�ȷֱ�Ϊa mol/L��b mol/L�������Sԭ���غ��֪��SO2��ʼ���ʵ���Ũ��Ϊ��a��b��mol/L����Ӧ����ͨ���õ�λʱ����Ũ�ȵı仯������ʾ����������SO3�Ļ�ѧ��Ӧ����Ϊ
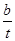 mol/(L��min)��
mol/(L��min)����2�������ڴ����ڷ�Ӧǰ�䣬��˸����ܷ�Ӧʽ�͵�һ����Ӧʽ��֪���ܷ�Ӧʽ��ȥ��һ����Ӧʽ���õ��ڶ�����Ӧʽ�����ڶ�����ӦʽΪ4Fe2����O2��4H����4Fe3����2H2O
��������Һ�к��������ӣ����������ͭ���Ʊ��������Ҫ�������ӳ�ȥ�����Ե�����ҺpH��Ŀ�ľ��ǽ�Fe3+ת��ΪFe(OH)3������ȥ������Ϊ�ڳ�ȥ�����ӵ�ͬʱ�������������µ����ʣ���˲��� ѡ���������ƺͰ�ˮ��Ӧ��ѡ������ͭ���ʽ̼��ͭ������ѡbc��
��3������֪��Ӧ��TiO2(s)��2Cl2(g)��TiCl4(l)��O2(g) ��H����140 kJ��mol��1����2C(s)��O2(g)��2CO(g) ��H����221 kJ��mol��1������ݸ�˹���ɿ�֪�٣��ڼ��õ���ӦTiO2(s)��2Cl2(g)��2C(s)��TiCl4(l)��2CO(g)�����Ը÷�Ӧ�ķ�Ӧ�Ȧ�H��140 kJ��mol��1��221 kJ��mol��1����81 kJ��mol��1��
�ڸ���TiCl4(l)��ȡ����TiO2�ķ���֮һ�ǽ�TiCl4���嵼�����������У�700��1000�棩����ˮ���֪��TiCl4(l)��ȡ����TiO2�Ļ�ѧ����ʽΪTiCl4+2H2O
 TiO2+4HCl��
TiO2+4HCl��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 CH3OH(g) ��H
CH3OH(g) ��H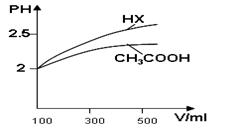

 CO2(g)+H2(g)���÷�Ӧ�ġ�H________0��ѡ�����������������������������900Kʱ���÷�Ӧƽ�ⳣ���Ķ���ֵ��lgK��=_____________.
CO2(g)+H2(g)���÷�Ӧ�ġ�H________0��ѡ�����������������������������900Kʱ���÷�Ӧƽ�ⳣ���Ķ���ֵ��lgK��=_____________.
 N������941kJ��������1molN4����ת��Ϊ2molN2ʱҪ�ų�______________ kJ������
N������941kJ��������1molN4����ת��Ϊ2molN2ʱҪ�ų�______________ kJ������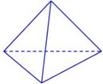
 ���߹�ϵ��ȷ����
���߹�ϵ��ȷ���� W (s) + 3H2O (g)
W (s) + 3H2O (g)  W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1
W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1  WI4 (g)������˵����ȷ����____________��
WI4 (g)������˵����ȷ����____________��