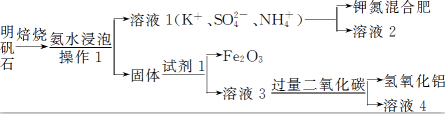
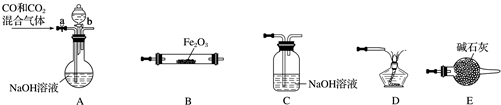
��Ŀ����
1��Fe��OH��2 �ܲ��ȶ���¶���ڿ��������ױ�������Fe��OH��2 ��������ѧ����ʽΪ4Fe��OH��2+O2+2H2O�T4Fe��OH��3��Ϊ�˻�ð�ɫ��Fe��OH��2 �����������ò���Fe3+ ��FeSO4 ��Һ���ò���O2 ������ˮ���Ƶ�NaOH ��Һ��Ӧ�Ʊ����ٳ�ȥ����ˮ���ܽ��O2 �����ü��ȷ�����
�ڼ���Fe2+ ���ڵ�����Լ������軯�� ����K3[Fe��CN��3]���������Dz���������ɫ����
�����ɰ�ɫFe��OH��2 �����IJ������ó��ι���ȡ����O2 ��NaOH ��Һ��������Һ�Ϸ��б����FeSO4 ��ҺҺ���£��ټ�����NaOH ��Һ�����������������Ƿ�ֹFe��OH��2 ��������
���� Fe��OH��2¶���ڿ��������ױ���������Fe��OH��3��Ϊ�˻�ð�ɫ��Fe��OH��2 ������Ӧ��ֹFe��OH��2��������
��Ϊ��ֹ������������������������ˮ�ų�������
�ڼ���Fe2+�����������Լ����軯�أ��ܲ���������ɫ������
�۳��ι���ȡ����O2 ��NaOH ��Һ��������Һ�Ϸ��б����FeSO4 ��ҺҺ���£��ټ�����NaOH ��Һ���������������
��� �⣺Fe��OH��2¶���ڿ��������ױ���������Fe��OH��3�������ķ�ӦΪ4Fe��OH��2+O2+2H2O�T4Fe��OH��3���ʴ�Ϊ��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��
�ٳ�ȥ����ˮ���ܽ��O2 �����ü��ȷ������ɷ�ֹ�������������������ʴ�Ϊ�����ȣ�
�ڼ���Fe2+ ���ڵ�����Լ������軯�� ����K3[Fe��CN��3]���������Dz���������ɫ�������ʴ�Ϊ�����軯�� ����K3[Fe��CN��3]���� ����������ɫ������
�����ɰ�ɫFe��OH��2 �����IJ������ó��ι���ȡ����O2 ��NaOH ��Һ��������Һ�Ϸ��б����FeSO4 ��ҺҺ���£��ټ�����NaOH ��Һ��������Һ�ϲ㣬������������֪���������������Ƿ�ֹFe��OH��2 ���������ʴ�Ϊ����ֹFe��OH��2 ��������
���� ���⿼�������������������Ʊ���Ϊ��Ƶ���㣬�����������������Ʊ�ԭ������ֹ�䱻����Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��342g ���ǣ�C12H22O11������1Lˮ�У�������Һ�����ʵ���Ũ��Ϊ1mol/L | |
| B�� | ��1L2mol/L H2SO4��Һ��ˮϡ�͵�2L��������Һ�����ʵ���Ũ��Ϊ1mol/L | |
| C�� | ��1L 8.4 mol/L��H2SO4��Һ���뵽1Lˮ�У�������Һ�����ʵ���Ũ��Ϊ9.2mol/L | |
| D�� | ��336mL HCl��������ˮ�����300 mL��Һ��������Һ�����ʵ���Ũ��Ϊ0.05mol/L |
| A�� | 11.2L��22.4L | B�� | 15.68L��17.92L | C�� | 13.44L��20.16L | D�� | 16.8L��16.8L |
| A�� | ������������ʴ���������� | |
| B�� | ����Ƭ�϶�ͭʱ��Ƭ������ | |
| C�� | ��ⱥ��ʳ��ˮʱ������������ | |
| D�� | ��п���巢���绯ѧ��ʴʱ���Ǹ��� |
| A�� | �������ὦ���۾��У�������ˮ��ϴ����ϴ��գ�۾� | |
| B�� | ������Ũ��Һ����Ƥ���ϣ������ô���ˮ��ϴ��Ȼ��Ϳ��������Һ | |
| C�� | ��������ʵ������ȼ�ŵľƾ���ʧ��������ʪ������ | |
| D�� | ����������Һʱ������Ͳ��ȡһ�������Ũ���ᣬ�ٱ߽��������Ͳ��������ˮ |
| A�� | ���������ܲ���H2����Һ�У����ܴ��ڴ�����Na+��Ba2+��AlO2-��NO3- | |
| B�� | SO2ͨ���ˮ�У���Ӧ�����ӷ���ʽΪSO2+I2+2H2O�TSO32-+2I-+4H+ | |
| C�� | 25��ʱNH4Cl��Һ��KW����100��ʱNaCl��Һ��KW | |
| D�� | 100��ʱ����pH=2��������pH=12��NaOH��Һ�������ϣ���Һ������ |
| A�� | K+��Na+��HCO3-��NO3- | B�� | Na+��K+��SO42-��Cl- | ||
| C�� | H+��Mg2+��SO42-��NO3- | D�� | Ag+��K+��NO3-��Na+ |
| A�� | ������ȡ��������ƽ������ | B�� | ��ȼ������֮ǰ��Ӧ���ȼ��鴿�� | ||
| C�� | ���ձ��ܽ��Ȼ��ƹ��� | D�� | ��ʵ���ҵ������Ƿ�����ζ |