��Ŀ����
����Ŀ����1�������������ڵ���ʵ���______���ܵ������_____������������ʵ���______(����д���)��
�ٴ�����Һ���Ҵ��۵����������Ȼ��Ƣݶ�������ް�ˮ�����ᱵ�����Ǣ�����������Ũ����
��2��Cl2ͨ��һ��Ũ��NaOH��Һ�����������ֺ��Ȼ�����,����n(NaClO):n(NaClO3)= 5:1,д��Cl2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ����˫���ű�ʾ������ת�Ƶķ������Ŀ:_________��
��3����״����4.48LCO2����100mL3.0mol/LNaOH��Һ,���ò���ɷ���_____,���ʵ���Ϊ________��
���𰸡� �ۢܢߢ� �٢ܢޢ� �� 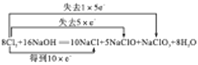 Na2CO3��NaHCO3 ��0.1mol
Na2CO3��NaHCO3 ��0.1mol
����������1���ٴ�����Һ�ǻ�������Ϊ������ʣ�������Һ�д��������ƶ������ӣ�������Һ������Ҵ�ֻ�����Ҵ����ӣ�û�������ƶ������ӣ����Բ��ܵ��磻�Ҵ��ǻ������ˮ��Һ�к�����״̬��ֻ�����Ҵ����ӣ�û�������ƶ������ӣ����Բ��ܵ��磬�Ƿǵ���ʣ��۵�����ͭ���Ӻ���������ӹ��ɣ�����û�������ƶ������ӣ����Բ����磻��ˮ��Һ�л�����״̬�£��ܵ���������ƶ���ͭ���Ӻ���������ӵ��磬�����ǵ���ʢ������Ȼ��ƣ��������ƶ��������Ӻ������ӣ��ܵ��磻���ǻ�������ڵ���ʣ��ݶ�������ֻ���ڷ��ӣ�û�������ƶ������ӣ����Բ��ܵ��磻����������ˮ��Һ����ˮ��Ӧ���������ᣬ���������������ƶ������ӵ��磬���������������ܵ��룬���������Ƿǵ���ʣ��ް�ˮ�ǻ��������������ƶ������ӣ��ܵ��磻�����ᱵ��û�������ƶ������Ӳ����磻 ��Ȼ���ᱵ��ˮ�е��ܽ�Ⱥ�С�������ᱵֻҪ�ܽ����ȫ���룬�������ᱵ�ǵ���ʣ�������ֻ�������Ƿ��ӣ�û�������ƶ������ӣ����Բ��ܵ��磻�����ǻ������ˮ��Һ�к�����״̬��ֻ�������Ƿ��ӣ�û�������ƶ������ӣ����Բ��ܵ��磬�Ƿǵ���ʣ�����������û�������ƶ������Ӳ����磬�ܽ���ˮ�IJ��ֵ��룬����������ʣ���Ũ�����ǻ�������Ϊ������ʣ�������Һ�д��������ƶ������ӣ�������Һ���� �����ڵ���ʵ��Ǣۢܢߢ����ܵ������ �٢ܢޢ�������������ʵ��Ǣ��2����NaClO3��NaClO1��5����д������ƽ����ʽ��8Cl2��16NaOH=NaClO3��5NaClO��10NaCl��8H2O�� ����3����״����4.48LCO2���ʵ���Ϊ4.48L/22.4L��mol��1=0.2mol��100mL3.0mol/LNaOH�����ʵ���Ϊ100��10-3L��3.0mol��L��1=0.3mol���ɷ���ʽ2NaOH��CO2=Na2CO3��H2O ��NaOH��CO2=NaHCO3��n(NaOH)/n(CO2)=2/3������2��1֮�䣬���Բ���ΪNa2CO3��NaHCO3�Ļ��������ʵ����ֱ�Ϊx��y����Na�غ㣺2x+y=0.3mol��x+y=0.2mol�����x=y=0.1mol��Na2CO3��NaHCO3���ʵ���Ϊ0.01mol��
����3����״����4.48LCO2���ʵ���Ϊ4.48L/22.4L��mol��1=0.2mol��100mL3.0mol/LNaOH�����ʵ���Ϊ100��10-3L��3.0mol��L��1=0.3mol���ɷ���ʽ2NaOH��CO2=Na2CO3��H2O ��NaOH��CO2=NaHCO3��n(NaOH)/n(CO2)=2/3������2��1֮�䣬���Բ���ΪNa2CO3��NaHCO3�Ļ��������ʵ����ֱ�Ϊx��y����Na�غ㣺2x+y=0.3mol��x+y=0.2mol�����x=y=0.1mol��Na2CO3��NaHCO3���ʵ���Ϊ0.01mol��

����Ŀ���±���25 ��ʱijЩ����ĵ���ƽ�ⳣ����
��ѧʽ | CH3COOH | HClO | H2CO3 | H2C2O4 |
Ka | Ka��1.8��10��5 | Ka��3.0��10��8 | Ka1��4.1��10��7 Ka2��5.6��10��11 | Ka1��5.9��10��2 Ka2��6.4��10��5 |
��1��H2C2O4�뺬�����ʵ�����KOH����Һ��Ӧ��������Һ�����ԣ�����Һ�и�����Ũ���ɴ�С��˳��Ϊ__________��
��2��pH��ͬ��NaClO��CH3COOK��Һ������Һ�����ʵ���Ũ�ȵĴ�С��ϵ��CH3COOK________NaClO������Һ�У�[c(Na��)��c(ClO��)]________[c(K��)��c(CH3COO��)](����>����<����������)��
��3����0.1 mol��L��1 CH3COOH��Һ�еμ�NaOH��Һ��c(CH3COOH)��c(CH3COO��)��5��9����ʱ��ҺpH��________��
��4��ȡ10 mLpH��2��CH3COOH��Һ������������ˮ�����ƹ���(����������ǰ����Һ������ֲ���)���������ܽ����Һ��![]() �ı�ֵ��________(��������������С��������ȷ����)��
�ı�ֵ��________(��������������С��������ȷ����)��
��5����̼������Һ�еμ�������ˮ�����ӷ���ʽΪ____________________��
����Ŀ�����и�������֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ� ��
ѡ�� | a | b | C | m |
A | �������� | ̼���� | ̼������ | ������̼ |
B | ���� | ������ | �������� | �� |
C | ������ | �������� | ƫ������ | �������� |
D | ���� | һ������ | �������� | ���� |

A. A B. B C. C D. D


