��Ŀ����
����Ŀ��I�������仯�����������������ϵ���С�
(1)��ͼ��ʵ�����о���ˮ����բ��ͬ��λ��ʴ���������ʾ��ͼ.

���õ绯��ʴ��Ϊ___________��
��ͼ��A��B��C��D�ĸ�������������������___________ (����ĸ)��
(2)��֪��Fe(s)+O2(g)=FeO(s)H=-272.0kJmol-1
C(s)+O2(g)=CO2(g)����H=-393.5kJmol-1
2C(s)+O2(g)=2CO(g)����H=-221kJmol-1
���¯����������FeO(s)+CO![]() Fe(S)+CO2(g) ��H=____________��
Fe(S)+CO2(g) ��H=____________��
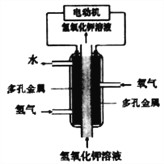
II���״���һ����Ҫ�Ļ���ԭ�Ϻ�����ȼ�ϡ���ͼ�Ǽ״�ȼ�ϵ�ع�����ʾ��ͼ������A��B��D��Ϊʯī�缫��CΪͭ�缫������һ��ʱ��Ͽ�K����ʱA��B�����ϲ��������������ͬ��


(1)���и����ĵ缫��ӦʽΪ_________________��
(2)����A�������������ڱ�״���µ����Ϊ_____________��
(3)��װ����Һ�н��������ӵ����ʵ�����ת�Ƶ��ӵ����ʵ����仯��ϵ��ͼ����ͼ�����߱�ʾ����_____________���ӵı仯����Ӧ������Ҫʹ��װ���н���������ǡ����ȫ��������Ҫ_________________mL 5��0 molL-1 NaOH��Һ��
���𰸡�
I.(1)��������ʴ����B��(2)-11KJ��mol-1��
II.��CH3OH�D6e-��8OH-=CO32-��6H2O����2.24L����Fe2����280��
��������
���������I.(1)����ˮ��Һ���ǽ�ǿ������Һ�����������������ʴ���ʴ�Ϊ��������ʴ���������Ӵ�������ˮʱ������������ʴ�ij̶��������������࣬����B����������࣬�ʴ�Ϊ��B��
(2)��֪����Fe(s)+![]() O2(g)�TFeO(s)��H=-272kJmol-1��
O2(g)�TFeO(s)��H=-272kJmol-1��
��C(s)+O2(g)�TCO2(g)��H=-393.5kJmol-1��
��2C(s)+O2(g)�T2CO(g)��H=-221kJmol-1��
���ݸ�˹���ɣ���-�ۡ�![]() -���ɵã�
-���ɵã�
FeO(s)+CO(g)=Fe(s)+CO2(g)����
��H=-393.5kJmol-1-![]() ��(-221kJmol-1)-(-272kJmol-1)=-11kJmol-1���ʴ�Ϊ��-11 kJmol-1��
��(-221kJmol-1)-(-272kJmol-1)=-11kJmol-1���ʴ�Ϊ��-11 kJmol-1��
����(1)�״�ȼ�ϵ����ԭ��ط�Ӧ���״��ڸ���ʧ���ӷ���������Ӧ���缫��ӦΪ��CH3OH-6e-+8OH-=CO32-+6H2O���ʴ�Ϊ��CH3OH-6e-+8OH-=CO32-+6H2O��
(2)����һ��ʱ��Ͽ�K����ʱA��B�����ϲ��������������ͬ�������缫��Ӧ��BΪ��������Һ��ͭ���������������ӵõ����������������������������ʵ���ΪX����Һ��ͭ�������ʵ���Ϊ0.1mol���缫��ӦΪ��
Cu2++2e-=Cu��
0.1mol 0.2mol
2H++2e-=H2����
2x x
A�缫Ϊ��������Һ�е�����������ʧ���������������缫��ӦΪ��
4OH--4e-=2H2O+O2����
4x x
���ݵ�ʧ�����غ�õ�0.2+2x=4x��x=0.1mol������A�����������������������ʵ���Ϊ0.1mol���ڱ�״���µ����Ϊ2.24L���ʴ�Ϊ��2.24L��
(3)����ת�Ƶ��ӵ����ʵ����ͽ��������ӵ����ʵ����ı仯����֪��ͭ���Ӵ������࣬���������ʵ�����С�������������ӣ�����ΪFe3+����ΪFe2+����ΪCu2+����ͼ��֪����ת��Ϊ0.4mol������Cu2+���ʵ���Ϊ0.2mol�������缫��ӦFe3++e-=Fe2+����Ӧ��������Һ����Fe2+Ϊ0.5mol��Cu2+Ϊ0.2mol��������Ҫ����NaOH��Һ0.5��2+0.2��2=1.4mol����������NaOH��Һ�����Ϊ![]() =0.28L=280mL���ʴ�Ϊ��Fe2+��280��
=0.28L=280mL���ʴ�Ϊ��Fe2+��280��

����Ŀ����1��105 Pa��298 Kʱ����1 mol��̬AB���ӷ������̬Aԭ�Ӻ�Bԭ������Ҫ��������Ϊ����(kJ��mol��1)��������һЩ���ۼ��ļ��ܣ�(��֪���������������ȼ۵ĵ���ۼ�)
���ۼ� | H-H�� | N��N�� | N-H�� |
����(kJ��mol��1) | 436 | 945 | 391 |
��ҵ�ϳɰ��Ļ�ѧ����ʽ��N2��3H2![]() 2NH3��
2NH3��
�Ͽ�1 mol N2�еĻ�ѧ����_____ (����ա��ų���)945 kJ�������γ�2 mol NH3�еĻ�ѧ����_____ (����ա��ų���) ________ kJ������
��298 Kʱ��ȡ1 mol N2��3 mol H2����һ�ܱ������У��ڴ��������½��з�Ӧ�������Ϸų������յ�����ΪQ1����Q1Ϊ____________ kJ��
�����ϱ��е������жϹ�ҵ�ϳɰ��ķ�Ӧ��______(����ȡ����ȡ�)��Ӧ��