��Ŀ����
��Ҫ��ش��������⣺
( 1��ij�л����һ�����Ӻ� 50 �����ӡ� �� ����Ϊ���������ķ���ʽ����Ϊ______�� �� ����Ϊ��̼���⡢�����л�����ķ���ʽ������_____________________����ͬһ��̼ԭ������������ һOH �Ľṹ���ȶ�������ÿ������ֻ��һ�֣�
( 2 �������£���� M ��ˮ��Һ���������������ϲ����������ܷ�����Ӧ��
X2 + Y2![]() 2XY , M�Ļ�ѧʽ������__________________________��
2XY , M�Ļ�ѧʽ������__________________________��
( 3 �����ϡ������I ) �������II��![]() �����III��+�������IV )���Ļ�ѧ����ʽ��_______________________________________________________________
�����III��+�������IV )���Ļ�ѧ����ʽ��_______________________________________________________________
( 4 ��ij������ X �к��ƺ����������Ϊ 23 : 16 ���������ᷴӦ�ų����壬д�� X ��ϡ���ᷴӦ�����ӷ���ʽ��_______________________________________________��
��1�� �� C6H14 , C7H8�� �� C5H10O , C4H10O2��C3H8O3 ��
( 2 ) HCl �� NaCl��MgCl2��AlC13 �Ȼ��ý����Ȼ������һ�֣�
( 3 )SiO2 + CaCO3![]() CaSiO3ʮ CO2����
CaSiO3ʮ CO2����
��SiO2 + Na2CO3 ![]() NaZSiO3CO2��
NaZSiO3CO2��
(4�� S2һʮ2H+===H2S���� SO32һʮ2H+=== SO2 ��ʮ H2O ��ÿ�� 2 �֣�
����:
( 1 �����л���Ϊ���� 50 / 8==6 �� 2 �����ķ���ʽΪ C6H14���滻������һ��̼������ 6 ���⣬�÷���ʽΪ C7H8����ױ���C6H5һCH3�����������к���һ����ԭ�ӣ���һ����ԭ�ӵĵ�������һ�� CH2 �������൱���������ķ���ʽΪ C5H12O���촼�����������к���������ԭ�ӣ��� 2 �������һ�� CH2CH2 �����ķ���ʽΪ C4H10O2���������ƣ� C3H8O3����HOCH2CH (OH )CH2OH .
( 2 ���������������Ϊ1 : 1 ������������Ϊ����������������ҺΪ���ᡢ���ý����Ȼ������Һ�� M Ϊ�Ȼ��⡢�Ȼ��Ƶȡ�
( 3 �����ݡ����¡�������ѧ��ѧ�л�ѧ��Ӧ��Ȼ������������ɸѡ��
( 4 �������⣬ X ��������ԭ����֮��Ϊ 2 : 1 ���� X ������ Na2S��Na2SO3��N a2SO4�����������Ʋ������ᷴӦ��

 �Ķ��쳵ϵ�д�
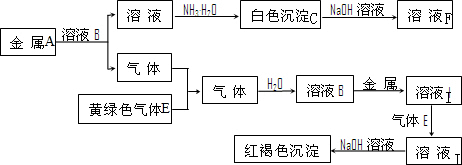
�Ķ��쳵ϵ�д� A��B��C��D�����ɶ�����Ԫ����ɵij������ʣ�����A��B��C����ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ��ʾ�����ֲ�������ȥ����
A��B��C��D�����ɶ�����Ԫ����ɵij������ʣ�����A��B��C����ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ��ʾ�����ֲ�������ȥ���� H++CN-��H2O
H++CN-��H2O H++OH-��CN-+H2O
H++OH-��CN-+H2O HCN+OH-
HCN+OH-