��Ŀ����
ijѧ����̽��FeSO4��Һ��ŨHNO3�ķ�Ӧ��
��ͬѧ��ʢ��FeSO4��Һ���Թ��е�������Ũ���ᣬ�����Թܣ�Ԥ������Ϊ�Թ��л�������������ɫ���壬��Һ��ɫ��ơ���ʵ�ʲ���ʱ�۲쵽Һ���Ϸ�����仯����ɫ�����Թ�����Һ��ɫ��Ϊ����ɫ��
Ϊ�˽�һ��̽����Һ��Ϊ����ɫ��ԭ��ͬѧ��������ʵ�顣
�ش���������
(1)��FeSO4��Һ�ͷ�Ӧ����Һ�м���KSCN��Һ��ǰ�߲����ɫ�����߱�죬������Ľ�����________��
(2)��ͬѧͨ���������ϣ���Ϊ��Һ������ɫ������NO2��NO����Һ��Fe2+��Fe3+������Ӧ���õ��ġ�Ϊ����������ͼװ�ã��������Ѽ��飬β������װ���ԣ�����̽����

��.����a���ر�b����ʹ��װ���з�Ӧ��ʼ�۲쵽������Һ��Ϊ����ɫ����������Һ�����Ա仯��
��.����b���ر�a��һ��ʱ�����ֹͣ���з�Ӧ��
��.Ϊ�����ʵ����ж������¸�����������ʹ���з�Ӧ�ظ����в����ʵ�飬�۲쵽�������벽�������ͬ��
��ͭ������Ũ���ᷴӦ�����ӷ���ʽ��_______________��
��װ���ҵ��Լ�Ϊ____________________��
�۲�����Ŀ����_______________________��
�ܸ�ʵ��ɵó��Ľ�����______________________��
(3)��ͬѧ���½���FeSO4��Һ��ŨHNO3�ķ�Ӧ��ʵ�飬�۲쵽��Ԥ��������ʵ�������_________����Ӧ�����ӷ���ʽΪ___________________
���в������ܴﵽĿ�ĵ���
ѡ�� | Ŀ�� | ���� |
A | ����100mL1.0mol/LCuSO4��Һ | ��25.0gCuSO4��5H2O����100mL����ˮ�� |
B | ��ȥNaCl������������Na2CO3 | �����������ˮ���ټ����Թ��������ᣬ�ټ��������ᾧ |
C | ʹNa2SO3��Һ�е�SO32-��ȫת��ΪSO42- | ��Na2SO3��Һ�м��������H2O2��Һ |
D | ȷ��KI��Һ�еĵ������Ƿ������� | ȡ������Һ���Թ��У������еμ�����������Һ���۲���Һ�Ƿ����ɫ������ȡ������Һ�μ�AgNO3��Һ���۲���Һ�Ƿ������ɫ���� |
A. A B. B C. C D. D
��ҵ����ú��ˮΪԭ��ͨ��һϵ��ת���ɱ�Ϊ�����Դ������ҵԭ�ϼ״���
��1����֪��C(s)+O2(g)=CO2(g) ��H1
��2H2(g)+O2(g)=2H2O (l) ��H2
��H2O (l)= H2O (g) ��H3
��̼��ˮ������ӦC(s)+2H2O(g) CO2(g)+2H2(g)�Ħ�H =________��
CO2(g)+2H2(g)�Ħ�H =________��
��2����ҵ��Ҳ���Խ�����������Ӧ�õ���CO2��H2��һ���ϳɼ״�����Ӧ����ʽΪ��CO2(g)��3H2(g) CH3OH(g)��H2O(g����H��0
CH3OH(g)��H2O(g����H��0
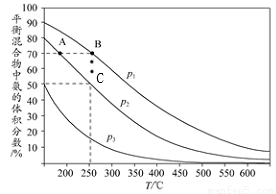
�ٹ�ҵ����������CO2��H2��ת����________���ǰ�ߴ������ߴ���һ�������жϡ�����Ϊ����״��IJ��ʿ��Բ�ȡ�Ĵ�ʩ��_______________�������㣩��
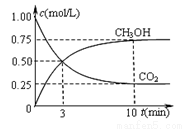
����һ���º����ܱ������г���1 mol CO2��3 mol H2����������Ӧ�����CO2��CH3OH(g)Ũ����ʱ��仯����ͼ��ʾ�����¶��µ�ƽ�ⳣ��Ϊ______��������λ��Ч���֣���
�ı��¶ȣ�ʹ��ӦCO2(g)+3H2(g) CH3OH(g)+H2O(g)�е��������ʶ�Ϊ��̬����ʼ�¶������ͬ��T1�桢2 L�ܱ�����������Ӧ�����в������ݼ��±���
CH3OH(g)+H2O(g)�е��������ʶ�Ϊ��̬����ʼ�¶������ͬ��T1�桢2 L�ܱ�����������Ӧ�����в������ݼ��±���
��Ӧʱ�� | CO2��mol�� | H2��mol�� | CH3OH��mol�� | H2O��mol�� | |
��Ӧ�� ���º��� | 0min | 2 | 6 | 0 | 0 |
10min | 4.5 | ||||
20min | 1 | ||||
30min | 1 | ||||
��Ӧ�� ���Ⱥ��� | 0min | 0 | 0 | 2 | 2 |
�ٴﵽƽ��ʱ����Ӧ��Աȣ�ƽ�ⳣ��K(��)___K(��)�����������������=������ͬ����ƽ��ʱCH3OH��Ũ��c(��)___c(��)��
�ڶԷ�Ӧ��ǰ10 min�ڵ�ƽ����Ӧ���ʦ�(CH3OH)=______����30 minʱֻ���������ٳ���1 mol CO2(g)��1 mol H2O(g)����ƽ��_____�ƶ��������������������