��Ŀ����
������ɫ��CuSO4��Һ����μ��백ˮʱ���۲쵽����������ɫ������������������ܽ��Ϊ����ɫ����Һ��������ɫ��Һ��ͨ��SO2���壬�������˰�ɫ����������ɫ��������ϡ�����У��������˺�ɫ��ĩ״�����SO2���壬ͬʱ��Һ����ɫ����������ʵ����������Ʋ⣬����������ȷ���� �� ��


A��Cu2+��Ag+���ƣ�����NH3�������ͭ�������� | B����ɫ����Ϊ+2��ͭ��ij���������Σ�����H2SO4�������ֽⷴӦ | C����ɫ����Ϊ+1��ͭ��ij���������Σ������������£�ֻ������������Ӧ | D����Ӧ���������ĵ�SO2�����ɵ�SO2�����ʵ������ |
A
 ��ɫ�������ڰ�ˮ��������ɫ����Һ��ΪCu(NH3)42+����ɫ��������ϡ�����У��������˺�ɫ��ĩ״�����SO2���壬�ʷ�����������ԭ��Ӧ��SO2��Ҫ�к����İ�ˮ�������ĵ�SO2�����ɵ�SO2�����ʵ�����������ȡ�
��ɫ�������ڰ�ˮ��������ɫ����Һ��ΪCu(NH3)42+����ɫ��������ϡ�����У��������˺�ɫ��ĩ״�����SO2���壬�ʷ�����������ԭ��Ӧ��SO2��Ҫ�к����İ�ˮ�������ĵ�SO2�����ɵ�SO2�����ʵ�����������ȡ�
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
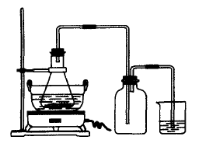
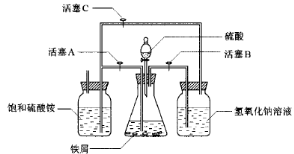
 ��1����Ӧ������ͭ���ٿˣ�
��1����Ӧ������ͭ���ٿˣ� H2SO4�����ʵ���֮��Ϊ �� ��
H2SO4�����ʵ���֮��Ϊ �� ��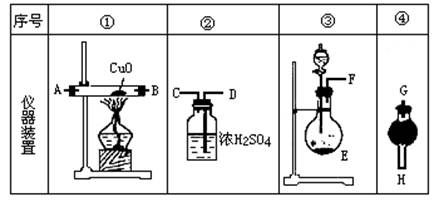

 ��
��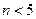 ��m<10}�������룬����PFS��ˮ�����γ���״������������ӡ����������Ϣ������˵������ȷ����
��m<10}�������룬����PFS��ˮ�����γ���״������������ӡ����������Ϣ������˵������ȷ����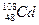 ������������Ϊ60
������������Ϊ60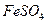 ��Һ��PFS�辭��������ˮ��;ۺϵĹ���
��Һ��PFS�辭��������ˮ��;ۺϵĹ���