��Ŀ����
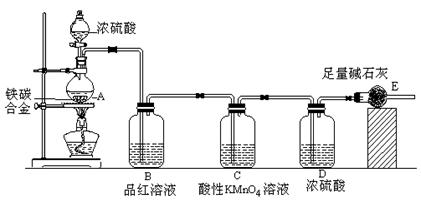
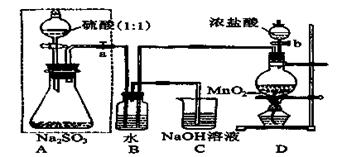
��ͼ�У�A�������ķ���װ�ã�B��C�Ǿ��������װ�ã�D��װ��˿������Ӧ��E�ĵײ�����ɫ����ۼ���F�����ն��������װ�á�
��1������װ������һ��������ָ���� ��������ĸ��ʾ����
��2��ͨ��B��Ϊ�˳�ȥ ����B��Ӧ���� ��ͨ��C��Ϊ�˳�ȥ ��
��3��д��D��F�з�����Ӧ�Ļ�ѧ����ʽ �� ��
��4�����A�в�������3.36L����״����������㣺
��д��A�з�����Ӧ�Ļ�ѧ����ʽ�������A�еĵ���ת����� ��
������MnO2�����ʵ��� ���۱�������HCl�����ʵ��� ��
��1��B
��2��HCl ����ʳ��ˮ ˮ����(ˮ) ����1�֣�
��3��2Fe+3Cl2 2FeCl3 Cl2+2NaOH=NaCl+NaClO+H2O ����2�֣�
2FeCl3 Cl2+2NaOH=NaCl+NaClO+H2O ����2�֣�
��4������2�֣�
�ٱ����ת�Ƶ����� ˫���Ŷ����ԡ�
��0.15mol ��0.3mol
���������������1��B�н����ܺͳ�����Ӧ�Ե�����2���Ʊ��������к���HCl��ˮ���������ʣ�Ӧ���ñ���ʳ��ˮ��ȥHCl������Ũ�����ȥˮ������
3mol��0.15mol ��
���㣺����������Ʊ��������й����⡣

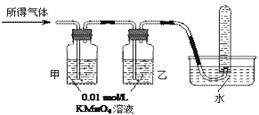
��ҵ�ϳ�����������ʢװ��Ũ���ᡣij��ȤС���ͬѧ���֣���һ������������Ũ�������ʱ���۲쵽������ȫ�ܽ⣬�������������塣ʵ�������������Լ��� 0.01 mol/L ����KMnO4��Һ��0.10 mol/L KI��Һ��������ˮ��������Һ������ˮ������Э������̽��������Һ������ijɷ֡�
��������롿
��������Һ�еĽ������ӿ��ܺ���Fe2����Fe3���е�һ�ֻ����֣�
�����������п϶����� ���塣
��ʵ��̽����
| | ʵ����� | Ԥ������ | �� �� |
| ��֤����� | ����٣�ȡ����0.01 mol/L ����KMnO4��Һ������������Һ�� | | |
| ����ڣ� | | ����Fe3�� | |
| ��֤����� | ����������ͨ������װ�� | | �������ֻ��������� |
���������ۡ�
��1����ͬѧ�����������ѡ��KSCN��Һ���������KSCN��������ˮ������Һ������ɲ���������̽���������Ƿ���У���˵��ԭ�� ��
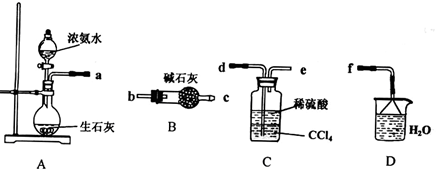
��2����ͬѧ������Թ������������H2��Q���壬Ϊ�����������ʵ��װ������ͼ��ͼ�мг�����ʡ�ԣ���
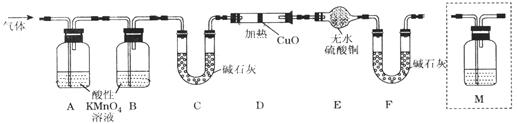
��Q������ԭ���� ���û�ѧ����ʽ��ʾ����
��Ϊȷ��Q�Ĵ��ڣ���M��ʢ�ŵ��Լ�Ϊ ������װ��M������ ��ѡ����ţ���
a��A֮ǰ b��A-B�� c��B-C�� d��C-D��
��װ��ͼ��D��E��F��ϵ������� ��
ij�о�С��̽����
I ��ͭƬ��Ũ����ķ�Ӧ���г�װ�ú�A�м���װ������,�������Ѽ��飩
II�� SO2�� Fe( NO3)3��Һ�ķ�Ӧ[1.0 mol/L�� Fe(NO3)3��Һ�� pH��1]��ش������й����⣺
̽��I
(l)ijѧ����������ʵ��:ȡ12.8gͭƬ��20 mL 18 mol?L-1��Ũ�����������ƿ�й���,ֱ����Ӧ���,�������ƿ�л���ͭƬʣ�࣬ͬʱ������ѧ��֪ʶͬѧ����Ϊ���н϶������ʣ�ࡣ
��װ��A�з�Ӧ�Ļ�ѧ����ʽ��_______
�ڸ�ͬѧ���������Ũ�ȵ�ʵ�鷽���Dzⶨ��������������䷽���ж���,�������з����в����е���______ (����ĸ����
| A�������������建��ͨ��Ԥ�ȳ�����ʢ�м�ʯ�ҵĸ����,������Ӧ���ٴγ��F |
| B�������������建��ͨ�������{�������Һ,�ټ�������BaCl2��Һ�����ˡ�ϴ�ӡ������������ |
| C������ˮ���ⶨ�������������(����ɱ�״���� |
| D�����ű���NaHSO3��Һ�ķ����ⶨ�������������(����ɱ�״���� |
(2)Ϊ�ų�������ʵ��ĸ��ţ��μ�Ũ����֮ǰӦ���еIJ�����______��
(3)�b��B�в����˰�ɫ����������B�в�����ɫ������ԭ������������ֲ��룺
����1:SO2��Fe3+��Ӧ������2 ��������������SO2��NO3-��Ӧ������3��____________��
�ٰ�����1��װ��B�з�Ӧ�����ӷ���ʽ��______��֤���ò���Ӧ��һ��ȷ�����ɵ������ʣ���ʵ�������������____________��
�ڰ�����2,ֻ�轫װ��B�е�Fe(NO3)3��Һ�滻Ϊ�����������ij����Һ������ͬ�����½���ʵ�顣Ӧѡ����滻��Һ��______ (����ţ���
a��0.1 mol/L ϡ���� b�� 1.5 mol/L Fe(NO3)2��Һ
c�� 6.0 mol/L NaNO3��0.2 mol/L����������ϵ���Һ