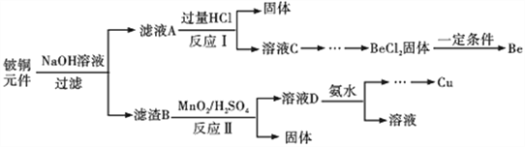
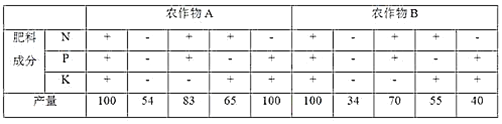
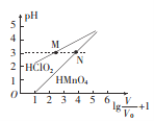
��Ŀ����
����Ŀ���й����ʵ����ļ�������ѧ��ѧ����Ҫ���֣���ش������й����ʵ����ļ������⡣
��1���ڱ�״���£�67.2 L CO2��__________mol������Ϊ_______g������__________��CO2���ӣ����к���__________mol��ԭ�ӡ�
��2���ڱ�״���£�1.7 g������ռ�����ԼΪ_________L������ͬ������_____mol H2S������ͬ����ԭ������
��3������CO��CO2��O3��������,���Ƿֱ���1 mol O,��������������ʵ���֮��Ϊ_________��
��4����״����,11.2 L X������ӵ�����Ϊ16 g,��X�����Ħ��������___________��
���𰸡�3.0 132 3NA 6 2.24 0.15 6��3��2 32 g/mol
��������
(1)�ڱ�״���£�67.2 L CO2�����ʵ���Ϊ![]() =3mol������Ϊ44g/mol��3mol
=3mol������Ϊ44g/mol��3mol
=132g������3NA��CO2���ӣ����к���3mol��2=6mol��ԭ�ӣ�
(2)n(NH3)=![]() =0.1mol��V(NH3)=0.1mol��22.4L/mol=2.24L��n(H)=0.3mol����n(H2S)=0.15mol��
=0.1mol��V(NH3)=0.1mol��22.4L/mol=2.24L��n(H)=0.3mol����n(H2S)=0.15mol��
(3)CO��CO2��O3�������壬���Ǻ��е���ԭ�Ӹ���֮��Ϊ1:2:3������������������ԭ�ӵ����ʵ�����Ϊ1mol����n(CO)=1mol��n(CO2)=![]() mol��n(O3)=
mol��n(O3)=![]() mol������������������ʵ���֮��Ϊ1:
mol������������������ʵ���֮��Ϊ1:![]() :
:![]() =6:3:2��
=6:3:2��
(4)��״���£�11.2 L X��������ʵ���Ϊ![]() =0.5mol�����������Ϊ16 g����X�����Ħ������Ϊ
=0.5mol�����������Ϊ16 g����X�����Ħ������Ϊ![]() =32g/mol��
=32g/mol��