��Ŀ����
��12�֣�1 Lij�����Һ�����ܺ��е��������±���
(1)������Һ����μ���NaOH��Һ���ʵ����ȣ����� ��������������ʵ�����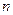 �������NaOH��Һ�������
�������NaOH��Һ�������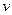 ���Ĺ�ϵ����ͼ��ʾ��
���Ĺ�ϵ����ͼ��ʾ��
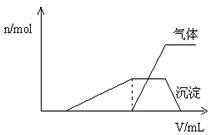
�����Һ��ȷ�����е�������__ __ ___ __��
__ ___ __��
����ȷ���Ƿ��е���������_______ ___��
Ҫȷ������ڿɲ�������ʵ����______ _��
�϶������ڵ���������________ ___��
��2������⣬����Һ�к��д����� Cl�� ��Br����I��������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl����Br����I�������ʵ�����ͨ��Cl2�������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
Cl�� ��Br����I��������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl����Br����I�������ʵ�����ͨ��Cl2�������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
�ٵ�ͨ��Cl2�����Ϊ2��8 Lʱ����Һ�з�����Ӧ�����ӷ���ʽΪ__ _��
��ԭ��Һ��Cl����Br����I�������ʵ���Ũ��֮��Ϊ_____ ________��
| ���ܴ������е������� | H+ NH4+ Al3+ K+ |
| ���ܴ������е������� | Cl- Br- I? ClO? AlO2- |
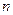 �������NaOH��Һ�������
�������NaOH��Һ�������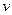 ���Ĺ�ϵ����ͼ��ʾ��
���Ĺ�ϵ����ͼ��ʾ��
�����Һ��ȷ�����е�������__
 __ ___ __��
__ ___ __������ȷ���Ƿ��е���������_______ ___��
Ҫȷ������ڿɲ�������ʵ����______ _��
�϶������ڵ���������________ ___��
��2������⣬����Һ�к��д�����
 Cl�� ��Br����I��������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl����Br����I�������ʵ�����ͨ��Cl2�������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
Cl�� ��Br����I��������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl����Br����I�������ʵ�����ͨ��Cl2�������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺| Cl2���������״���� | 2��8L | 5��6 L | 11��2 L |
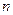 ��Cl���� ��Cl���� | 1��25mol | 1��5 mol | 2 mol |
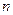 ��Br���� ��Br���� | 1��5 mol | 1��4 mol | 0��9 mol |
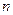 ��I���� ��I���� | 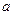 mol mol |  0 0 | 0 |
��ԭ��Һ��Cl����Br����I�������ʵ���Ũ��֮��Ϊ_____ ________��

��

��ϰ��ϵ�д�
�����Ŀ


 �Ÿ�ȡ���������Լ��ֱ����5֧�Թ��У�
�Ÿ�ȡ���������Լ��ֱ����5֧�Թ��У� ������������ˮ�����Թܣ��۲��� ����������ʵĻ�ѧʽ��
������������ˮ�����Թܣ��۲��� ����������ʵĻ�ѧʽ��  ����Һ�����Cʱ�����а�ɫ��������.
����Һ�����Cʱ�����а�ɫ��������.
 ����
����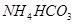 ����
����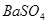 ����HF����
����HF����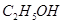 ����
����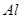 ����ʳ��ˮ����
����ʳ��ˮ����
 ��Һ��Ӧ����_____________��
��Һ��Ӧ����_____________�� ��ij����Һ������Ϊ���ᡢ���ᡢ�����е�һ�֣�Ϊ��ȷ��������Һ����ɽ���ʵ�飺ȡ
��ij����Һ������Ϊ���ᡢ���ᡢ�����е�һ�֣�Ϊ��ȷ��������Һ����ɽ���ʵ�飺ȡ ����
���� ��Һ����μ���
��Һ����μ��� _______________��
_______________�� ��
��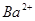 ��
��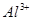 ��
��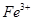
 ��
��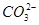 ��
��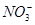 ��
��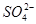
 ���£�
���£� Mn��CN��2 + ��CN��2�� + 2H2O
Mn��CN��2 + ��CN��2�� + 2H2O KI + ��CN��2
KI + ��CN��2 +fCO32��+bCl��+cH2O��������ѧ����ʽ���ܵ���ƽϵ���ж��飬��ش�
+fCO32��+bCl��+cH2O��������ѧ����ʽ���ܵ���ƽϵ���ж��飬��ش� ��1/2 C��2 D����
��1/2 C��2 D����