��Ŀ����
(8��)��5ƿ��ɫ�����Լ����ֱ���Ba(NO3)2��KCl��NaOH��CuSO4��Na2SO4����ֻ�ṩ����ˮ��ͨ�������ʵ�鲽��ɼ������ǡ�����д���пհף�
 �Ÿ�ȡ���������Լ��ֱ����5֧�Թ��У�
�Ÿ�ȡ���������Լ��ֱ����5֧�Թ��У� ������������ˮ�����Թܣ��۲��� ����������ʵĻ�ѧʽ��
������������ˮ�����Թܣ��۲��� ����������ʵĻ�ѧʽ��
 �Ʒֱ�ȡδ���Һ�������м��������Ѽ������Һ���۲쵽���������Ӧ�����ӷ���ʽ��
�Ʒֱ�ȡδ���Һ�������м��������Ѽ������Һ���۲쵽���������Ӧ�����ӷ���ʽ��
 �������Թ����а�ɫ�������ɣ����ӷ���ʽ�� ��
�������Թ����а�ɫ�������ɣ����ӷ���ʽ�� ��
 �������Թ�������ɫ�������ɣ����ӷ���ʽ�� ��
�������Թ�������ɫ�������ɣ����ӷ���ʽ�� ��
 �Ÿ�ȡ���������Լ��ֱ����5֧�Թ��У�
�Ÿ�ȡ���������Լ��ֱ����5֧�Թ��У� ������������ˮ�����Թܣ��۲��� ����������ʵĻ�ѧʽ��
������������ˮ�����Թܣ��۲��� ����������ʵĻ�ѧʽ��  �Ʒֱ�ȡδ���Һ�������м��������Ѽ������Һ���۲쵽���������Ӧ�����ӷ���ʽ��
�Ʒֱ�ȡδ���Һ�������м��������Ѽ������Һ���۲쵽���������Ӧ�����ӷ���ʽ�� �������Թ����а�ɫ�������ɣ����ӷ���ʽ�� ��
�������Թ����а�ɫ�������ɣ����ӷ���ʽ�� �� �������Թ�������ɫ�������ɣ����ӷ���ʽ�� ��
�������Թ�������ɫ�������ɣ����ӷ���ʽ�� �� �����������Լ� ���ѧʽ�����Ѽ������������ѡ����������δ��������ʡ�
�����������Լ� ���ѧʽ�����Ѽ������������ѡ����������δ��������ʡ�
��

��ϰ��ϵ�д�
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�
�����Ŀ
 ϡ�����У���Һ�� ���ӵ������٣� ���ӵ������ӣ� ���ӵ���û�б仯.
ϡ�����У���Һ�� ���ӵ������٣� ���ӵ������ӣ� ���ӵ���û�б仯.  �ӵ�Ũ�����±�����M���ӿ���Ϊ:
�ӵ�Ũ�����±�����M���ӿ���Ϊ: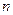 �������NaOH��Һ�������
�������NaOH��Һ�������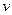 ���Ĺ�ϵ����ͼ��ʾ��
���Ĺ�ϵ����ͼ��ʾ��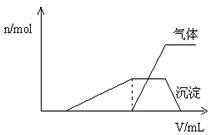
 __ ___ __��
__ ___ __�� Cl�� ��Br����I��������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl����Br����I�������ʵ�����ͨ��Cl2�������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
Cl�� ��Br����I��������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl����Br����I�������ʵ�����ͨ��Cl2�������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺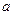 mol
mol