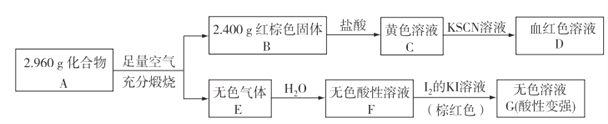
��Ŀ����
����Ŀ��I. ���ʯ��ʯī��Ϊ̼��ͬ�������壬����ȼ��ʱ����������������һ����̼�������ȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ��

��1���������Ľ��ʯ��ʯī��ȫȼ�գ�___________(�������ʯ���� ��ʯī��)�ų����������࣬д����ʾʯīȼ���ȵ��Ȼ�ѧ����ʽ��______________________________________��
��2����ͨ��״���£�__________________(�������ʯ������ʯī��)���ȶ���д��ʯīת��Ϊ���ʯ���Ȼ�ѧ����ʽ��__________��
��3��12 gʯī��һ����������ȼ�գ��������� 36 g���ù����зų�������Ϊ_______ ��
II. ij��Ӧ��������Ӧ A��B��C ���ɣ����ķ�Ӧ����������ͼ��ʾ(E1��E2��E3��E4 ��ʾ���)���ش��������⡣
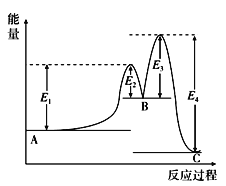
��1��A��B �����е� ��H______________(����������������)0��
��2���������_____________________(������������������)�ı䷴Ӧ���ʱ䡣
��3��������Ӧ�� ��H��_____________________________________________��
���𰸡� ���ʯ C(ʯī��s)+ O2(g)![]() CO2(g) ��H=-393.5 kJmol-1 ʯī C(ʯī��s)
CO2(g) ��H=-393.5 kJmol-1 ʯī C(ʯī��s)![]() C(���ʯ��s) ��H=+1.9 kJmol-1 252 kJ �� ���� E1+E3-E2-E4
C(���ʯ��s) ��H=+1.9 kJmol-1 252 kJ �� ���� E1+E3-E2-E4
����������1������ͼ���֪�������Ľ��ʯ��ʯī��Ƚ��ʯ�������ߣ�������ȫȼ�ս��ʯ�ų�������������1molʯī��ȫȼ�շų���������110.5kJ+283.0kJ��393.5kJ�����Ա�ʾʯīȼ���ȵ��Ȼ�ѧ����ʽΪC(ʯī��s)+ O2(g)��CO2(g) ��H=-393.5 kJmol-1����2���������Ľ��ʯ��ʯī��Ƚ��ʯ�������ߣ�������ͨ��״����ʯī���ȶ���ʯīת��Ϊ���ʯ������395.4kJ��393.5kJ��1.9kJ������ʯīת��Ϊ���ʯ���Ȼ�ѧ����ʽΪC(ʯī��s)![]() C(���ʯ��s) ��H=+1.9 kJmol-1����3��12 gʯī��1mol����ȫת��ΪCO2��44g����ȫת��ΪCO��28g�������������� 36 g����˵���������ǻ�����壬�����ɵ�CO2��CO�ֱ���xmol��ymol����x+y��1��44x+28y��36�����x��y��0.5�����Ըù����зų�������Ϊ0.5mol����110.5kJ/mol+393.5 kJ/mol����252kJ��
C(���ʯ��s) ��H=+1.9 kJmol-1����3��12 gʯī��1mol����ȫת��ΪCO2��44g����ȫת��ΪCO��28g�������������� 36 g����˵���������ǻ�����壬�����ɵ�CO2��CO�ֱ���xmol��ymol����x+y��1��44x+28y��36�����x��y��0.5�����Ըù����зų�������Ϊ0.5mol����110.5kJ/mol+393.5 kJ/mol����252kJ��
II.��1��A��B �����з�Ӧ���������������������������������H��Ӧ����0����2����������ܸı��ܣ������ܸı䷴Ӧ���ʱ䡣��3������ͼ���Ͽ�֪������Ӧ�� ��H����(E4��E1�DE3��E2)��

 ������������ϵ�д�
������������ϵ�д�