��Ŀ����
4������480mL 0.5mol•L-1��NaOH��Һ���Իش��������⣺��1��ѡ����������ɱ�ʵ��������������У�������ƽ����ȷ��0.1g����ҩ�ס��ձ�������������ͷ�ιܡ�500 mL����ƿ�ȣ�
��2�����㣺��ҪNaOH���������Ϊ10.0 g��
��3���������ٳ���������NaOH����Ӧ����С�ձ��в�������ƽ�����̣�����̡������̡�����
��ijѧ������������ƽ����һ��С�ձ�������������ǰ��������ڱ�ߵ���̶ȴ�����ƽ��ֹʱ����ָ���ڷֶ��̵�ƫ��λ�ã�˵����ʱ�������С�ڣ�����ڡ���С�ڡ����ұߣ��ٶ����ճƵ�С�ձ�������Ϊ32.6 g���32.6g����32.61g���������á�������ʾ�������Ϸ������룬��������ʾ��������ȡ������ij������̣�������ͼ�б���ϻ��������λ�ã�����������ʾ����
| ��������/g | 50 | 20 | 20 | 10 | 5 |
| ������ȡ��������̣� |

��4���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò������������ǽ�������ܽ⣮
��5��ת�ơ�ϴ�ӣ���ת��ʱӦʹ�ò�����������ϴ���ձ�2�Ρ�3����Ϊ�˱�֤����ȫ��ת��������ƿ�У�
��6�����ݡ�ҡ�ȣ����ݵIJ�����������ƿ�м�������ˮ����̶���1 cm��2 cmʱ�����ý�ͷ�ιܵμ�����ˮ��Һ��İ�Һ����ʹ��Ϳ̶������У�
��7��ijѧ��ʵ������NaOH��Һ��Ũ��Ϊ0.48mol•L-1��ԭ�������A��C������ĸ����
A��ʹ����ֽ�����������ƹ���
B������ƿ��ԭ��������������ˮ
C���ܽ����ձ�δ�����ϴ�ӣ�
���� ��1����������һ�����ʵ���Ũ�ȵ���Һ����ѡ��������Ȼ���жϻ�ȱ�ٵ�������
��2������m=CVM�������ʵ�������
��3��������������ƽʹ�÷���������������
��������ƽ�ľ�ȷ��Ϊ0.1g���ֶ��̵�ָ��ƫ�ҽ����ƽ�ij���ԭ������ȷʹ�÷������
��4���ܽ�����ʹ�ò��������Լӿ��ܽ����ʣ�
��5����ҺʱΪ��ֹҺ��������Ӧ�ò�����������ϴ���ձ�2�Ρ�3����Ϊ�˱�֤����ȫ��ת��������ƿ�У�
��6�����ݶ��ݵ���ȷ�������
��7�������������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1������480mL 0.5mol•L-1��NaOH��Һ��Ӧѡ��500ml����ƿ��ʵ�������Ƶ���500mL 0.5mol/L������������Һ�����Ʋ���Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���Ҫʹ�õ������У�������ƽ�����������ձ���ҩ�ס�500mL����ƿ����ͷ�ιܵȣ���ȱ�ٵIJ�������Ϊ��500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��2������480mL 0.5mol•L-1��NaOH��Һ����Ҫ����������������=0.5L��0.5mol•L-1��40g/mol=10.0g��
�ʴ�Ϊ��10.0g��
��3���ٳ���������NaOH����Ӧ����С�ձ��в�������ƽ�����̣�
�ʴ�Ϊ�����̣�
�ڷֶ��̵�ָ��ƫ�ң�˵���ұ��أ����̸������̣���������С�����̣�������ƽ�ľ�ȷ��Ϊ0.1g������
С�ձ�������32.6 g��
��������ƽȷ�����������������̼���50g���룬��������ʾΪ��50g������Ȼ��20g���룬�����������㣬��ʾΪ��20g�����ټ�20g���룬20g������������ʾΪ����������10g���룬10g�����������㣬��ʾΪ��10g�����ټ�5g���룬5g����������������5g���룬��ʾΪ��5g����������ƶ����뵽2.6g���е�ƽ��ƽ������ϻ��������λ��Ϊ�� ��
��
�ʴ�Ϊ�����̡�С�ڡ�32.6 g��
| ��������/g | 50 | 20 | 20 | 10 | 5 |
| ������ȡ��������̣� | ���� | �� | ���� | �� | ���� |
 ��
����4���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò������������ǣ����裬�����ܽ⣻
�ʴ�Ϊ�����裬�����ܽ⣻
��5����ҺʱΪ��ֹҺ��������Ӧ�ò�����������ϴ���ձ�2�Ρ�3����Ϊ�˱�֤����ȫ��ת��������ƿ�У�
�ʴ�Ϊ������������֤����ȫ��ת��������ƿ�У�
��6�����ݵIJ����ǣ�������ƿ�м�������ˮ����̶���1 cm��2 cmʱ�����ý�ͷ�ιܵμ�����ˮ��Һ��İ�Һ����ʹ��Ϳ̶������У�
�ʴ�Ϊ��������ƿ�м�������ˮ����̶���1 cm��2 cmʱ�����ý�ͷ�ιܵμ�����ˮ��Һ��İ�Һ����ʹ��Ϳ̶������У�
��7��A��ʹ����ֽ�����������ƹ��壬���³�����������������ƫС�����ʵ���ƫС����Һ��Ũ��ƫ�ͣ���Aѡ��
B������ƿ��ԭ��������������ˮ�������ʵ����ʵ�������Һ�������������Ӱ�죬��Һ��Ũ�Ȳ��䣬��B��ѡ��
C���ܽ����ձ�δ�����ϴ�ӣ��������ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ���Cѡ��
��ѡ��AC��
���� ���⿼��������һ�����ʵ���Ũ����Һ����ȷ����ԭ�������ǽ���ؼ���ע������ƿ��������ƽ��ʹ�÷�������Ŀ�ѶȲ���

��Cl- ��Fe2+ ��Fe3+ ��S ��H+ ��Na+ ��Mg��
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢݢ� | D�� | �ڢܢݢ� |
��CCl4 ��NH3 ��CH4 ��CO2 ��N2 ��H2S ��SO2 ��CS2 ��H2O ��HF��
| A�� | �ڢۢܢݢ� | B�� | �٢ۢܢݢ� | C�� | �٢ۢܢ� | D�� | ���Ͼ����� |
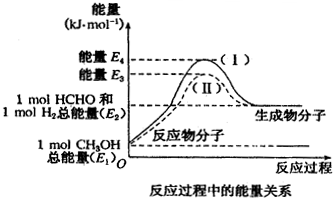
 ��1����25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55kJ��������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��CH4��g��+2O2��g���TCO2��g��+H2O��l����H=-880kJ/mol��
��1����25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55kJ��������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��CH4��g��+2O2��g���TCO2��g��+H2O��l����H=-880kJ/mol��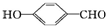 �ķе��
�ķе�� �ߣ�ԭ��
�ߣ�ԭ��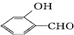 �γɷ������������
�γɷ������������ �γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������
�γɷ��Ӽ���������Ӽ����ʹ���Ӽ����������� ʵ��С��ͬѧ����һ����ij��ֽ���۳���Ư��Һ��NaCl��NaClO�Ļ��Һ������ʢ�ű���KAl��SO4��2��Һ�ij��У�����ж��¼�����С��ͬѧΪ̽���ж�ԭ�����������ʵ�飺
ʵ��С��ͬѧ����һ����ij��ֽ���۳���Ư��Һ��NaCl��NaClO�Ļ��Һ������ʢ�ű���KAl��SO4��2��Һ�ij��У�����ж��¼�����С��ͬѧΪ̽���ж�ԭ�����������ʵ�飺