��Ŀ����
(8��) ��1.92gͭ����һ������Ũ�����У����ȣ�����ͭ�IJ��ϼ��٣���Ӧ���ɵ�������ɫ��dz����ͭ��Ӧ���ʱ��ͭƬ��ȫ��ʧ�������ռ���1.12L���壨��״��������
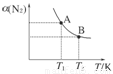
��1�����������У��йط�Ӧ�Ļ�ѧ����ʽΪ ��
��
��2����Ӧ�б���ԭ��HNO3�����ʵ����� mol��
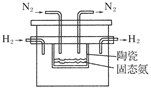
��3�����ռ��������������������ʢ��ˮ��ˮ���У��������л���ͨ��O2��ʹ���ַ�Ӧ����Ҫʹˮǡ�ó�����������������Ҫ�μӷ�Ӧ��O2�����ʵ����Ƕ���Ħ����
��8�֣���1��Cu+4HNO3(Ũ)= Cu(NO3)2+2NO2��+2H2O
3Cu+8HNO3= 3Cu(NO3)2+2NO2��+4H2O ����1�֣� ��2��0.05��2�֣�
��3��0.015 mol
����

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ
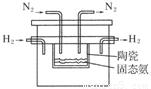
 ��Դ�Ŀ����������������Ŀɳ����Է�չϢϢ��أ�
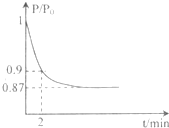
��Դ�Ŀ����������������Ŀɳ����Է�չϢϢ��أ� 2NH3(g)����֪298 Kʱ��
2NH3(g)����֪298 Kʱ��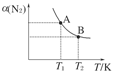
 )
) 2NH3(g) ��H=��92.4kJ/mol
2NH3(g) ��H=��92.4kJ/mol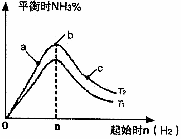

 2NH3(g)����֪298 Kʱ��
2NH3(g)����֪298 Kʱ��