��Ŀ����
����Ŀ�����ɽ��������������е�Ӧ��ʮ�ֹ㷺��
��.CrԪ���γɵ��Ȼ�����(CrO2Cl2)����Ҫ��;�����л��ϳ��п������������Ȼ��������������л��ﷴӦ��
(1)CrO2Cl2������Ϊ���ɫҺ�壬����CCl4��CS2�Ȼ��ܣ�������ʵ����ʵ�����ж�CrO2Cl2������_____________(���������������Ǽ�����)���ӣ��÷��ӵ����幹�͵�����Ϊ___________��
��.Cu���γɶ�����������������Ϣ�ش����⣺
(2)��ʢ������ͭˮ��Һ���Թ�����μ��백ˮ�����ȳ�����ɫ�����������μӰ�ˮ����ɫ�����ܽ⣬�õ�����ɫ������Һ���Ⱥ��������ӷ���ʽΪCu2++2NH3.H2O=Cu(OH)2��+2NH4+��_______________��
(3)��������ɫ����Һ�����Ҵ�����������ɫ�ľ��塣����ɫ����Ļ�ѧʽΪ__________________________�����������ԭ����_______________________________________________________������Cu���ʵķ�ĩ����NH3��Ũ��Һ�У�ͨ��O2����ַ�Ӧ����Һ������ɫ���÷�Ӧ�����ӷ���ʽ��____________________��
(4)��������ʵ����̣��ж�NH3��H2O��Cu2+����λ������NH3____H2O(������������������������С����)��
��.Ni(CO)6Ϊ��������ṹ�����е���ԭ��λ��������������ģ���λ��CO��������������������������ϡ�
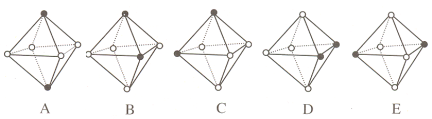
(5)������������CO��λ�廻�� NH3 �õ��µ����������������л�Ϊͬ���칹�����_________��(����ĸ��ţ�����һ�顣ͼ�кڵ�ΪNH3��ԲȦΪCO��Ni��ȥ)
���𰸡��Ǽ��� ƽ���ı��� Cu(OH)2+4NH3=[Cu(NH3)4]2++2OH-��Cu(OH)2+4NH3��H2O =[Cu(NH3)4]2++2OH- +4H2O [Cu(NH3)4]SO4.H2O [Cu(NH3)4]SO4���Ҵ��е��ܽ��ԶС����ˮ�е��ܽ�� 2Cu +8NH3��H2O+O2 = 2[Cu(NH3)4]2+ + 4OH��+ 6H2O ���� AE��BCD��������ϣ�������ͬ����������
��������
(1)CCl4��CS2��Ϊ�Ǽ��Է��ӣ�CrO2Cl2����CCl4��CS2�Ȼ��ܣ������������ܿ�֪��CrO2Cl2Ϊ�Ǽ��Է��ӣ���CrO2Cl2Ϊ������ṹ������Oԭ�Ӻ�Clԭ�ӶԵ�������������ͬ�����ӵ�����������IJ������غϣ�ӦΪ���Է��ӣ���ʵ����CrO2Cl2Ϊ�Ǽ��Է��ӣ�˵����Ϊƽ���ı��νṹ��������ԭ�ӶԳƣ�������ԭ�ӶԳƣ�
(2)��ˮ������ͭ��Ӧ����������ͭ��ɫ����������ˮ����ʱ����ˮ��������ͭ��Ӧ���ɿ����Ե�ͭ�����������������ܽ�õ�����ɫ������Һ���漰�����ӷ���ʽΪ��Cu2++2NH3H2O=Cu(OH)2��+2NH4+��Cu(OH)2+4NH3=[Cu(NH3)4]2++2OH-��Cu(OH)2+4NH3��H2O =[Cu(NH3)4]2++2OH- +4H2O��
(3)��������ɫ����Һ�����Ҵ�������[Cu(NH3)4]SO4���Ҵ��е��ܽ��С����ˮ�е��ܽ�ȣ����Ի���������ɫ�ľ��壺Cu(NH3)4SO4H2O������Cu���ʵķ�ĩ����NH3��Ũ��Һ�У�ͨ��O2����ַ�Ӧ����Һ������ɫ��˵������ͭ�������ӣ���ϵ����غ��Ԫ���غ�ɵ����ӷ���ʽΪ2Cu +8NH3��H2O+O2 = 2[Cu(NH3)4]2+ + 4OH��+ 6H2O��
(4)������ͭ��Һ�м��������ˮ��������[Cu(NH3)4]2+���ӣ�˵��������Cu2+����λ��������ˮ��ͭ���ӵ���λ������
(5)ͬ���칹���У�������������λ��ƽ���ı������ڡ����λ�û�������������������ϣ����Ի�Ϊͬ���칹����У�A��B(��A��C��A��D��B��E��C��E��D��E��һ�����)��