��Ŀ����
(11��)Ǧ�����ǵ��͵Ŀɳ��͵�أ����������������Ƕ��Բ��ϣ�����ܷ�ӦʽΪ��
Pb+PbO2+4H++2
 2PbSO4+2H2O
2PbSO4+2H2O
������������⣨�������⡢����������ԭ��:
(1)�ŵ�ʱ�������ĵ缫��Ӧʽ��__________________;���Һ��H2SO4��Ũ�Ƚ���__________________;�����·ͨ��1 mol����ʱ�������ϸ��������������_________________g��
(2)����ȫ�ŵ�ľ�PbO2��Pbʱ��������ͼ���ӣ����һ��ʱ�������A�缫������_________________��B�缫������_________________,��ʱǦ���ص��������ļ��Խ�_________________��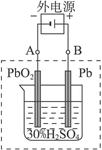
Pb+PbO2+4H++2

 2PbSO4+2H2O
2PbSO4+2H2O������������⣨�������⡢����������ԭ��:
(1)�ŵ�ʱ�������ĵ缫��Ӧʽ��__________________;���Һ��H2SO4��Ũ�Ƚ���__________________;�����·ͨ��1 mol����ʱ�������ϸ��������������_________________g��
(2)����ȫ�ŵ�ľ�PbO2��Pbʱ��������ͼ���ӣ����һ��ʱ�������A�缫������_________________��B�缫������_________________,��ʱǦ���ص��������ļ��Խ�_________________��

��1��PbO2+2e-+4H++2 ====PbSO4+2H2O С 48
====PbSO4+2H2O С 48
��2��Pb PbO2 �Ի�
 ====PbSO4+2H2O С 48
====PbSO4+2H2O С 48��2��Pb PbO2 �Ի�
���⿼��ԭ��غ͵��ص�ԭ����д���缫��Ӧʽ������ת�Ƶĵ���������ӵ����������ݷŵ�ͳ���ǿ���Ĺ��̿��жϣ�2���е���������

��ϰ��ϵ�д�
�����Ŀ

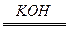 2H2O��Ӧ������ȼ�ϵ�أ���ͨ��Ӧ��____________������ͨ��Ӧ��____________���缫��ӦʽΪ��______________��______________��
2H2O��Ӧ������ȼ�ϵ�أ���ͨ��Ӧ��____________������ͨ��Ӧ��____________���缫��ӦʽΪ��______________��______________��
 AsO33-��I2��H2O �ݴ���Ƴ�����ͼ��ʾ��ʵ��װ��(װ�������ŵ�������ʹ����װ���γ�һ���պϻ�·)����(B)�ձ�����μ���Ũ���ᣬ��������ָ��ƫת
AsO33-��I2��H2O �ݴ���Ƴ�����ͼ��ʾ��ʵ��װ��(װ�������ŵ�������ʹ����װ���γ�һ���պϻ�·)����(B)�ձ�����μ���Ũ���ᣬ��������ָ��ƫת
