��Ŀ����
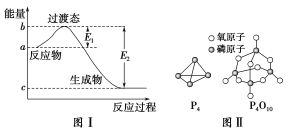
����Ŀ����ο�����ͼ������֪E1��134 kJ��mol��1��E2��368 kJ��mol��1������Ҫ��ش��������⣺
(1)ͼ����1 mol NO2(g)��1 mol CO(g)��Ӧ����CO2��NO�����е������仯ʾ��ͼ�����ڷ�Ӧ��ϵ�м����������Ӧ��������E1�ı仯��________(�������С�����䡱����ͬ)����H�ı仯��__________________________��
��д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��______________________________��
(2)�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧ���Ȼ�ѧ����ʽ���£�
��CH3OH(g)��H2O(g)===CO2(g)��3H2(g)
��H����49.0 kJ��mol��1
��CH3OH(g)��![]() O2(g)===CO2(g)��2H2(g)
O2(g)===CO2(g)��2H2(g)
��H����192.9 kJ��mol��1
��֪��H2O(g)===H2O(l) ��H����44 kJ��mol��1����״�����ȼ��ΪҺ̬ˮ���Ȼ�ѧ����ʽΪ______________________________��
(3)�±���ʾ�Dz��ֻ�ѧ���ļ��ܲ�����
��ѧ�� | P��P | P��O | O===O | P===O |
����/(kJ��mol��1) | a | b | c | x |
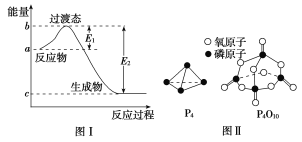
��֪����ȼ����Ϊd kJ��mol��1����������ȫȼ�յIJ���Ľṹ��ͼ����ʾ�������x��________kJ��mol��1(�ú�a��b��c��d�Ĵ���ʽ��ʾ)��
���𰸡�(1)��С ���� NO2(g)��CO(g)===CO2(g)��NO(g) ��H����234 kJ��mol��1
(2)CH3OH(g)��![]() O2(g)===CO2(g)��2H2O(l) ��H����764.7 kJ��mol��1
O2(g)===CO2(g)��2H2O(l) ��H����764.7 kJ��mol��1
(3) ![]() (d��6a��5c��12b)
(d��6a��5c��12b)
��������(1)�۲�ͼ��E1ӦΪ��Ӧ�Ļ�ܣ����������Ӧ�Ļ�ܽ��ͣ����Ǧ�H���䣻1 mol NO2(g)��1 mol CO(g)��Ӧ����CO2��NO�ķ�Ӧ����ֵ����Ӧ�����������������˸÷�Ӧ���Ȼ�ѧ����ʽΪNO2(g)��CO(g)===CO2(g)��NO(g)
��H����234 kJ��mol��1��
(2)�۲췽��ʽ�����ø�˹���ɣ��������Ȼ�ѧ����ʽ���������㣺�ڡ�3���١�2���ۡ�2����������״�����ȼ�յ��Ȼ�ѧ����ʽ��
(3)����ȼ�յĻ�ѧ����ʽΪP4��5O2![]() P4O10�����ͼ���а�������ȫȼ�ղ���Ľṹ�����ݡ���Ӧ�ȣ���Ӧ������ܺͣ�����������ܺ͡���ȼ���ȸ���ɵõ�ʽ��6a��5c��(4x��12b)����d���ݴ˿ɵ�x��
P4O10�����ͼ���а�������ȫȼ�ղ���Ľṹ�����ݡ���Ӧ�ȣ���Ӧ������ܺͣ�����������ܺ͡���ȼ���ȸ���ɵõ�ʽ��6a��5c��(4x��12b)����d���ݴ˿ɵ�x��![]() (d��6a��5c��12b)��
(d��6a��5c��12b)��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�