��Ŀ����
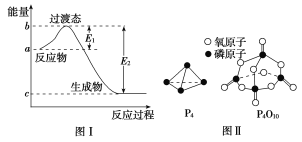
����Ŀ����֪E1��134 kJ/mol��E2��368 kJ/mol����ο�����ͼ������Ҫ����գ�
(1)ͼ����1 mol NO2(g)��1 mol CO(g)��Ӧ����CO2(g)��NO(g)�����е������仯ʾ��ͼ�����ڷ�Ӧ��ϵ�м����������Ӧ���ʼӿ죬E1�ı仯��________(���������С�����䡱����ͬ)����H�ı仯��________��NO2��CO��Ӧ���Ȼ�ѧ����ʽΪ��________________________��
(2)��̼����(��Ҫָ����CO2)�ڽ������������ŷ��о�����Ҫ�����á�ĿǰNH3��(NH4)2CO3�Ѿ���������ҵ��̼����������CO2�ɷ������¿��淴Ӧ��
��Ӧ��2NH3(l)��H2O(l)��CO2(g)![]() (NH4)2CO3(aq) ��H1
(NH4)2CO3(aq) ��H1
��Ӧ��NH3(l)��H2O(l)��CO2(g)![]() NH4HCO3(aq) ��H2
NH4HCO3(aq) ��H2
��Ӧ��(NH4)2CO3(aq)��H2O(l)��CO2(g)![]() 2NH4HCO3(aq) ��H3
2NH4HCO3(aq) ��H3
��H3�릤H1����H2֮��Ĺ�ϵ�ǣ���H3��________��
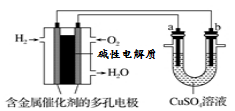
(3)�±���ʾ�Dz��ֻ�ѧ���ļ��ܲ�����
��ѧ�� | P��P | P��O | O===O | P===O |
����/kJ��mol��1 | a | b | c | x |
��֪����ȼ����Ϊd kJ/mol����������ȫȼ�����ɵIJ���Ľṹ��ͼ����ʾ������x��________ kJ��mol��1(�ú�a��b��c��d�Ĵ���ʽ��ʾ)��
���𰸡�(1)��С ����
NO2(g)��CO(g)===CO2(g)��NO(g) ��H����234 kJ/mol
(2)2��H2����H1
(3) ![]() (d��6a��5c��12b)
(d��6a��5c��12b)
��������(1)��������ܽ��ͷ�Ӧ����Ļ�ܣ���E1��E2����С����E2��E1���䣬����Ӧ�Ȳ��ı䣬��ͼ֪��1 mol NO2��1 mol CO��Ӧ����CO2��NO�ų�����368��134��234 kJ���÷�Ӧ���Ȼ�ѧ����ʽΪNO2(g)��CO(g)===NO(g)��CO2(g) ��H����234 kJ��mol��1��(2)�۲췽��ʽ�����ø�˹���ɣ��ڡ�2���ٵ÷���ʽ�ۣ��ʦ�H3��2��H2����H1��(3)����ȼ�յĻ�ѧ����ʽΪP4��5O2===P4O10��1 mol������ȫȼ�����6 mol P��P��5 mol O===O���γ�12 mol P��O��4 mol P===O����12 mol��b kJ��mol��1��4 mol��x kJ��mol��1��(6 mol��a kJ��mol��1��5 mol��c kJ��mol��1)��d kJ��mol��1�����x��![]() (6a��5c��d��12b) kJ��mol��1��
(6a��5c��d��12b) kJ��mol��1��

 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�����Ŀ���������ʷ������ȷ�����( )
����� | ������ | ���� | �� | |
A | NaOH��Һ[_K] | ���� | ʯī | ʳ�� |
B | ����ʯ��ˮ | KNO3���� | O3 | ���Na2CO3�� |
C | ���� | ʯ��ʯ | �� | ��ʯ�� |
D | CuSO4��5H2O | CaCl2 | ˮ�� | CaO |
A. A B. B C. C D. D