��Ŀ����
ij�л���A��C��H��O����Ԫ����ɣ���ȡ18g A������O2�г��ȼ�գ���ȼ�ղ���ȫ������ͨ����ˮ�Ȼ��ƺͼ�ʯ�Һ�����������������Ϊ10.8g��26.4g��
��1��A�����ʽ��______
��2����������A�����壬��A�Ľṹ��ʽ��______
��3����A����16��ʱ����ɫ���壬�������ԣ���A�ķ���ʽ��______
��4����0.1molA��9.6g�������ھƻ�ø����������ת���ɾƾ�����A������Ϊ______��
��1��A�����ʽ��______
��2����������A�����壬��A�Ľṹ��ʽ��______
��3����A����16��ʱ����ɫ���壬�������ԣ���A�ķ���ʽ��______
��4����0.1molA��9.6g�������ھƻ�ø����������ת���ɾƾ�����A������Ϊ______��
��ˮ�Ȼ������ص�����Ϊˮ��������n��H2O��=
=0.6mol����ʯ�����ص�����ΪCO2��������n��CO2��=
=0.6mol���������m��C��=0.6mol��12g/mol=7.2g��m��H��=1.2g��m��O��=18g-7.2g-1.2g=9.6g������18g������n��O��=
=0.6mol�����л�����N��C����N��H����N��O��=0.6��1.2��0.6=1��2��1�����ʽΪCH2O�����ʽΪCH2O���м�ȩ�����ᡢ�����ǵȣ�
��1�������Ϸ�����֪���л�������ʽΪCH2O���ʴ�Ϊ��CH2O��
��2����������A�����壬ӦΪ��ȩ���ṹ��ʽ��HCHO���ʴ�Ϊ��HCHO��
��3����A����16��ʱ����ɫ���壬�������ԣ�ӦΪ���ᣬ����ʽΪC2H4O2���ʴ�Ϊ��C2H4O2��
��4����0.1molA��9.6g������0.6mol��˵�������к���6��O������ʽΪC6H12O6�����ھƻ�ø����������ת���ɾƾ���ӦΪ�����ǣ�
�ʴ�Ϊ�������ǣ�
| 10.8g |
| 18g/mol |
| 26.4g |
| 44g/mol |
| 9.6g |
| 16g/mol |
��1�������Ϸ�����֪���л�������ʽΪCH2O���ʴ�Ϊ��CH2O��
��2����������A�����壬ӦΪ��ȩ���ṹ��ʽ��HCHO���ʴ�Ϊ��HCHO��
��3����A����16��ʱ����ɫ���壬�������ԣ�ӦΪ���ᣬ����ʽΪC2H4O2���ʴ�Ϊ��C2H4O2��
��4����0.1molA��9.6g������0.6mol��˵�������к���6��O������ʽΪC6H12O6�����ھƻ�ø����������ת���ɾƾ���ӦΪ�����ǣ�
�ʴ�Ϊ�������ǣ�

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ
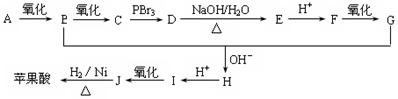 ��֪��
��֪��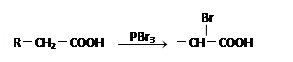
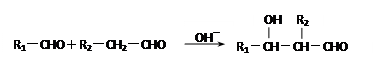
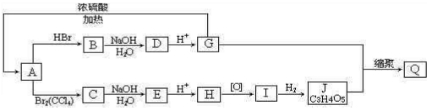
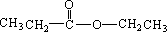 ����
����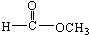 ��
��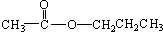 ����
����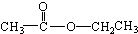 ��
��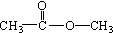
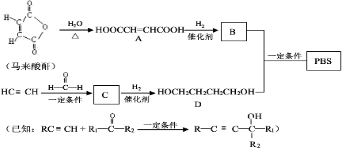
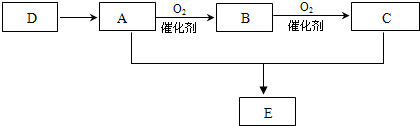
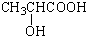 ������Է���������ȣ���������Na2CO3��Ӧ�ų�CO2��1molA���������Ʒ�Ӧ�ɲ���22.4LH2����״��������A�Ľṹ��ʽΪ______��______��
������Է���������ȣ���������Na2CO3��Ӧ�ų�CO2��1molA���������Ʒ�Ӧ�ɲ���22.4LH2����״��������A�Ľṹ��ʽΪ______��______��