��Ŀ����
��10�֣���֪A��B��C��D��E����Ԫ��Ϊǰ20��Ԫ�أ���ԭ��������������AԪ��������������������������ԭ����������ȣ�BԪ����AԪ���γɵ�һ��ʽ��Ϊ32�Ļ����ﳣ��������ƽ���������һ�ֻ������ˮ��Һ��ʹ��ɫʯ����ֽ������EԪ�ص�ԭ�Ӱ뾶��ǰ20��Ԫ���������ϡ�����壩��DԪ����AԪ�����γɷ���ʽΪAD���͵Ĺ��ۻ����CԪ�صĵ��ʺ���������ܼ�����AD��ˮ��Һ��Ҳ������EԪ������������Ӧ��ˮ����Իش�
��1��Ԫ�ط��ţ�A____ ��B_____ ��E ��
��2���Ƚ�C��D��E����Ԫ�ؼ����ӵİ뾶��С��ϵ�� �����ӷ��ű�ʾ��
��3������EԪ�ش��ڵķ����ǣ� ��
��4��д��CԪ�ص����������EԪ�ص�����������Ӧ��ˮ��������ӷ���ʽ��
��
��5��д��A��B��Ԫ���γɵ�ʽ��Ϊ32�Ļ�����ĵ���ʽ ����0.5mol����̬��������N2O4��g����ַ�Ӧ����������̬�������ų�������Ϊ269.175KJ����д���÷�Ӧ���Ȼ�ѧ��ʽ�� ��
��1��Ԫ�ط��ţ�A____ ��B_____ ��E ��
��2���Ƚ�C��D��E����Ԫ�ؼ����ӵİ뾶��С��ϵ�� �����ӷ��ű�ʾ��
��3������EԪ�ش��ڵķ����ǣ� ��
��4��д��CԪ�ص����������EԪ�ص�����������Ӧ��ˮ��������ӷ���ʽ��
��
��5��д��A��B��Ԫ���γɵ�ʽ��Ϊ32�Ļ�����ĵ���ʽ ����0.5mol����̬��������N2O4��g����ַ�Ӧ����������̬�������ų�������Ϊ269.175KJ����д���÷�Ӧ���Ȼ�ѧ��ʽ�� ��
��10�֣�(1)H N K (3��)
(2)Al3+?�� K+��Cl-��1�֣�
(3)ͨ����ɫ���ܲ���Ƭ�۲�����Ƿ�Ϊ��ɫ��1�֣�
(4) )Al3+ + 4OH- = AlO2-+2H2O��2�֣�
(5)��1�֣�
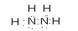
2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g������H=��1076.7kJ/mol��2�֣�
(2)Al3+?�� K+��Cl-��1�֣�
(3)ͨ����ɫ���ܲ���Ƭ�۲�����Ƿ�Ϊ��ɫ��1�֣�
(4) )Al3+ + 4OH- = AlO2-+2H2O��2�֣�
(5)��1�֣�
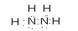
2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g������H=��1076.7kJ/mol��2�֣�
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
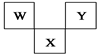
 ���ӵļ�����__________��
���ӵļ�����__________�� [Co(H2O)6]2+�Էۺ�ɫ������Co2+�ļ۵����Ų�ʽΪ________________________
[Co(H2O)6]2+�Էۺ�ɫ������Co2+�ļ۵����Ų�ʽΪ________________________ ����COCl2�����ں��� �����ţ���
����COCl2�����ں��� �����ţ��� Xԭ��������������˥���
Xԭ��������������˥���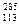 X�������й������У���ȷ����
X�������й������У���ȷ����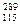 X��
X��