��Ŀ����
11����â����Na2SO4��10H2O���Ʊ���������[��NH4��2Al��SO4��2��12H2O]������������������ͼ��
��1����ҺC�е�������Ҫ��NH4HCO3
��2������淋���Һ�����ԣ�����刺����ھ�ˮ�������ӷ���ʽ˵����ԭ��Al3++3H2O?Al��OH��3+3H+
��3�����̢��еķ�Ӧ�¶Ȳ��ܳ���40�棬��ԭ����NH4HCO3�����ȷֽ�
��4�����û�ѧƽ��ԭ������Na2SO4�Թ�����ԭ��HCO32-��aq��+Na+��aq��?NaHCO3��s����Na+Ũ������ƽ��������Ӧ������
��5������Al2��SO4��3���뵽A�л���������ij��������壬��������淋IJ������ͣ��������ӷ���ʽ����
���Ͳ����������ԭ����Al3++3HCO32-=Al��OH��3��+3CO2��
��6����ҺE�е���������ΪNH4+��SO42-
��7����֪����淋��ܽ�������¶ȵ����߶������̢��еõ�����淋�ϵ��ʵ������ǣ�����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
���� ̼�������Һ�м��������ƣ����˵õ���������ҺA�����������յõ�̼�����������̼����֪����ΪNaHCO3������I�����ܽ�Ȳ�ͬ�������ֽⷴӦ��2NH4HCO3+Na2SO4=2NaHCO3��+��NH4��2SO4 ����ҺA�к��У�NH4��2SO4 ���ܽ��NH4HCO3���������ᣬ����pHʹNH4HCO3ת��������̼�루NH4��2SO4 ���õ���ҺBΪ��NH4��2SO4 ��Һ���ټ������������������D��ҪΪʣ���NH4HSO4��Al2��SO4��3��
��� �⣺̼�������Һ�м��������ƣ����˵õ���������ҺA�����������յõ�̼�����������̼����֪����ΪNaHCO3������I�����ܽ�Ȳ�ͬ�������ֽⷴӦ��2NH4HCO3+Na2SO4=2NaHCO3��+��NH4��2SO4 ����ҺA�к��У�NH4��2SO4 ���ܽ��NH4HCO3���������ᣬ����pHʹNH4HCO3ת��������̼�루NH4��2SO4 ���õ���ҺBΪ��NH4��2SO4 ��Һ���ټ������������������D��ҪΪʣ���NH4HSO4��Al2��SO4��3��
��1��CO2�백ˮ��Ӧ����NH4HCO3��NH4��2CO3���ʴ�Ϊ��NH4HCO3��
��2������Al3+��NH4+ˮ��ʹ��Һ�������ԣ�����刺����ھ�ˮ������Ϊ������ˮ��������������������£�Al3++3H2O?Al��OH��3+3H+���ʴ�Ϊ���Al3++3H2O?Al��OH��3+3H+��
��3��NH4HCO3�����ȷֽ⣬�ʷ�ӦӦ�����¶ȣ��ʴ�Ϊ��NH4HCO3�����ȷֽ⣻
��4����ƽ����ϵ������һ�ַ�Ӧ���Ũ�ȣ���Ӧ������У�������ⷴӦ���ת���ʣ�HCO32-��aq��+Na+��aq��?NaHCO3��s����Na+Ũ������ƽ��������Ӧ�����ƣ��ʴ�Ϊ��HCO32-��aq��+Na+��aq��?NaHCO3��s����Na+Ũ������ƽ��������Ӧ�����ƣ�
��5������˫ˮ�ⷴӦAl3++3HCO32-=Al��OH��3��+3CO2�����ʴ�Ϊ��Al3++3HCO32-=Al��OH��3��+3CO2����
��6��AΪ��NH4��2SO4��ʣ��������κ�̼���Σ�B��ҪΪNH4HSO4��D��ҪΪʣ���NH4HSO4��Al2��SO4��3��EΪ��NH4��2SO4����ҺE�е���������Ϊ��NH4+��SO42-���ʴ�Ϊ��NH4+��SO42-��
��7������淋��ܽ�������¶ȵ����߶����ʲ��ý��½ᾧ�ķ������룬����ҺŨ�ȵͣ�������ҪŨ��������Ϊ����Ũ�������½ᾧ����ȴ�ᾧ�����ʴ�Ϊ������Ũ������ȴ�ᾧ��
���� ���⿼���Ʊ�ʵ�鷽����ơ�������ɵIJⶨ����ȷ��������ԭ���ǽ���ؼ����ۺϿ���ѧ���������������������Ѷ��еȣ�

| A�� | Cu | B�� | Cu2O | C�� | CuS | D�� | Cu2S |
�ڳ����£��й����ʵ��ܽ��Ϊ��
| ���� | NH4Cl | NaHCO3 | Na2CO3 | NaCl |
| �ܽ��/g | 37.2 | 9.6 | 21.5 | 36.0 |
��2������I��II�ܷ�Ӧ�����ӷ���ʽΪNa++NH3+CO2+H2O�TNaHCO3��+NH4+��
��3������I��II���ܵߵ���ԭ������NH3�ڱ���NaCl��Һ���ܽ�����CO2����ͨNH3����Һ�ʼ��ԣ���������CO2������NaHCO3�����ɣ�����Ӧ��ͨ��NH3��
��4���������õ�̼���Ʒ�ĩ�Ƿ���NaHCO3����ʵ�鷽���ǣ�д���������衢�����ۣ���ȡ�����������Թܣ����ȣ�������������ͨ�����ʯ��ˮ����ʹ����ʯ��ˮ����ǣ�֤����NaHCO3��
��5��Ϊ�˲ⶨ����ȡ����Ĵ��ȣ���������ֻ��̼�����ƣ�����С���ʵ�鲽��Ϊ��
i��ʹ������װ����װʵ��װ�ã������������
ii����ȡWg��Ʒ����Cװ�õ���ƿ�У�����������ˮ�ܽ�
iii������Dװ�õ�����ΪW1 g
iv���ӷ�Һ©������ϡ���ᣬֱ�����ٲ�������Ϊֹ
v����a����������һ�����Ŀ������ٴγ���Dװ������ΪW2 g
vi���ظ�����v�IJ�����ֱ��Dװ�õ��������ٸı䣬�Ƶ�Dװ�õ�����ΪW3 g
��������ʵ��ش��������⣺
�ٵ�i����ʹ������װ�����ӵĽӿ�˳��Ϊ����b������e����f������c����d������g����h��[��h����g��]����i����
�ڵڶ���ʢ��ʯ��װ�õ������Ƿ�ֹ��������D�м�ʯ�Ҹ���ʵ�飮
�۲�����̼���ƺ�̼�����Ƶ����ʵ���֮��Ϊ$\frac{{w}_{3}-{w}_{1}}{44}$mol��
| A�� | CH3-CH=CH2�� | B�� | 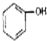 �� �� | C�� |  �� ��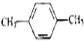 | D�� | ��������� |
��C��N��O��Al��Si��Cu�dz���������Ԫ�أ�
��1��Siλ��Ԫ�����ڱ��������ڵ�IVA�壮
��2��N�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p3��Cu�Ļ�̬ԭ���������1�����ӣ�
��3���á�����������գ�
| ԭ�Ӱ뾶 | �縺�� | �۵� | �е� |
| Al��Si | N��O | ���ʯ������� | CH4��SiH4 |
��4��H2O2�ĵ���ʽ
 ��
����5��þȼ�ղ�����CO2����û�ѧ����ʽ��ʾ������2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
��6����AgCl�����м���KBr��Һ����ɫ����ת��Ϊ����ɫ������д����Ӧ�����ӷ���ʽAgCl��s��+Br-�TAgBr��s��+Cl-��
��7���������������ԭ��Ӧ�����ӷ���ʽ��
2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
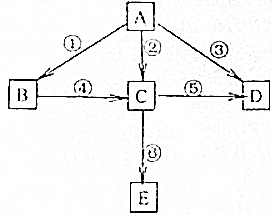 ��֪AΪ���ʣ�B��C��D��EΪ���������֮�������ͼת����ϵ��
��֪AΪ���ʣ�B��C��D��EΪ���������֮�������ͼת����ϵ�� ��
��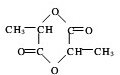 +2H2O��
+2H2O��