��Ŀ����
����Ŀ����һ���¶��£�10 mL 0.50 mol/L H2O2�������ֽ⡣��ͬʱ�̲ⶨ����O2�������������Ϊ��״�������±���
t/min | 0 | 2 | 4 | 6 | 8 | 10 |
v(O2��/mL | 0.0 | 9.9 | 17.2 | 22.4 | 26.5 | 29.1 |
������������ȷ���ǣ���Һ����仯���Բ��ƣ��� ��
A.0~6 min��ƽ����Ӧ���ʣ�v(H2O2)��0.033 mol/(L��min)
B.6~10 min��ƽ����Ӧ���ʣ�v(H2O2)��3.3��10-2 mol/(L��min)
C.��Ӧ��6 minʱ��H2O2�ֽ���40%
D.��Ӧ��10 minʱ��c(H2O2)��0.24 mol/L
���𰸡�B
��������
������Ӧ��2H2O2 ![]() 2H2O+O2��
2H2O+O2��
A.0��6minʱ���ڣ���������Ϊ![]() =0.001mol������c(H2O2)=0.001mol��2��0.01L=0.2mol/L������v(H2O2)=0.2mol/L��6min��0.033mol/(Lmin)����A��ȷ��
=0.001mol������c(H2O2)=0.001mol��2��0.01L=0.2mol/L������v(H2O2)=0.2mol/L��6min��0.033mol/(Lmin)����A��ȷ��
B�����ŷ�Ӧ�Ľ��У�H2O2��Ũ����С����Ӧ���ʼ�����6��10 min��ƽ����Ӧ����С��0��6minʱ���ڷ�Ӧ���ʣ���B����
C.6minʱ��H2O2�ֽ���Ϊ��![]() ��100%=40%����C��ȷ��
��100%=40%����C��ȷ��
D��0��10minʱ���ڣ���������Ϊ![]() =0.0013mol������c(H2O2)=0.0013mol��2��0.01L=0.26mol/L������10minʱc(H2O2)=0.5mol/L-0.26mol/L=0.24mol/L����D��ȷ��
=0.0013mol������c(H2O2)=0.0013mol��2��0.01L=0.26mol/L������10minʱc(H2O2)=0.5mol/L-0.26mol/L=0.24mol/L����D��ȷ��
�ʴ�ΪB��

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�����Ŀ�������й�ʵ���ѡ����ȷ���ǣ� ��
|
|
|
|
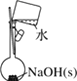
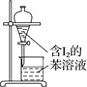
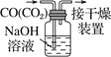
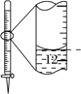
A.����0.10mol��L-1NaOH��Һ | B.����ȡ��ˮ��I2���ֳ�ˮ���IJ��� | C.��ȥCO�е�CO2 | D.��¼�ζ��յ����Ϊ12.20mL |
A.AB.BC.CD.D