��Ŀ����
A��B��C��D��E���ֶ�����Ԫ�أ�A��Dͬ���ڣ�A�ĵ��ʼȿ������ᷴӦ���ֿ���NaOH��Һ��Ӧ��B�ĵ����ڷŵ�����������������Ӧ��CԪ�ص����Ӳ������ӣ�DԪ��ԭ�ӵ��������������������������3/4��EԪ��ԭ�ӵ��������������������������3����
��1��A��ԭ�ӽṹʾ��ͼΪ ��
��2��0.1 mol��L A����������Һ��0.1 mol��L NaOH��Һ�������ϣ���Ӧ������
����ʽΪ ��
��3����A�ĵ��ʺ�NiO(OH)Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO(OH)ת��ΪNi(OH)2���õ���ܷ�Ӧ�Ļ�ѧ����ʽ�� ��
��4�����������Ԫ��A��B��ɣ��������õ��Ե�ԡ�
�����������ˮ������Ӧ���ɺ�B�Ļ������ң��ҷ����к���10�����ӡ�д����
��Ӧ�Ļ�ѧ����ʽ�� ��
��ҵ��A�ĵ��ʺͻ��������ڸ���1700K��Ӧ�Ʊ��ס���֪�÷�Ӧ������Ϊ����
����Ӧ���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��5��D��E����Ԫ����Ƚϣ��ǽ����Խ�ǿ����(��Ԫ������) ��������֤�ý��۵���(��д���) ��
a.�Ƚ�������Ԫ�صij������ʵķе�
b.�Ƚ�������Ԫ�صĵ������������ϵ�����
c.�Ƚ�������Ԫ�ص���̬�⻯����ȶ���
��6��C��D��E����γɱ��������ַ��ӣ����Ǿ�����18�����ӣ�����붡��Ӧ����D���ʵĻ�ѧ����ʽΪ ��
��1��A��ԭ�ӽṹʾ��ͼΪ ��
��2��0.1 mol��L A����������Һ��0.1 mol��L NaOH��Һ�������ϣ���Ӧ������
����ʽΪ ��
��3����A�ĵ��ʺ�NiO(OH)Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO(OH)ת��ΪNi(OH)2���õ���ܷ�Ӧ�Ļ�ѧ����ʽ�� ��
��4�����������Ԫ��A��B��ɣ��������õ��Ե�ԡ�
�����������ˮ������Ӧ���ɺ�B�Ļ������ң��ҷ����к���10�����ӡ�д����
��Ӧ�Ļ�ѧ����ʽ�� ��
��ҵ��A�ĵ��ʺͻ��������ڸ���1700K��Ӧ�Ʊ��ס���֪�÷�Ӧ������Ϊ����
����Ӧ���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��5��D��E����Ԫ����Ƚϣ��ǽ����Խ�ǿ����(��Ԫ������) ��������֤�ý��۵���(��д���) ��
a.�Ƚ�������Ԫ�صij������ʵķе�
b.�Ƚ�������Ԫ�صĵ������������ϵ�����
c.�Ƚ�������Ԫ�ص���̬�⻯����ȶ���
��6��C��D��E����γɱ��������ַ��ӣ����Ǿ�����18�����ӣ�����붡��Ӧ����D���ʵĻ�ѧ����ʽΪ ��
��1��
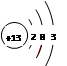 ��1�֣�
��1�֣���2��Al3+ + 3OH-�� Al(OH)3����2�֣�
��3��Al+3NiO(OH)+H2O+NaOH==NaAlO2+3Ni(OH)2��2�֣�
��4��AlN + 3H2O �� Al(OH)3��+ NH3����2�֣� 2Al + 2NH3
 2AlN + 3H2��2�֣�
2AlN + 3H2��2�֣���5������1�֣�bc ��2�֣� ��6��H2S+H2O2=S��+2H2O��2�֣�
���������A��B��C��D��EԪ�طֱ�ΪAl��N��H��S��O����2������������������������������������3��Ni���ϼ���+3�۱�Ϊ+2�ۣ������ϼ������Խ���������ƫ�����Σ��ʷ���ʽΪ Al+3NiO(OH)+H2O+NaOH==NaAlO2+3Ni(OH)2����4����ΪAlN��ˮ��ΪAl(OH)3��NH3����5���ǽ�����ǿ������ͨ�����������ϵ��������⻯����ȶ��ԡ�����������Ӧˮ��������ǿ���жϣ���6��H��S��O����Ԫ���γɵ�18���ӷ���ΪH2S��H2O2�������ܷ���������ԭ��Ӧ��

��ϰ��ϵ�д�
 ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ


 ��Һ�������
��Һ�������