��Ŀ����
��һ��ɫ��Һ������Na+��Ag+��Ba2+��Al3+��AlO2-��MnO4-��CO32-��SO42-�е������֡�ȡ����Һ��������ʵ�飺
��ȡ������Һ������������ᣬ���������ɣ����õ���Һ��
���ڢ�������Һ���ټ��������̼�������Һ�����������ɣ�ͬʱ������ɫ�����ס�
���ڢ�������Һ�м������ŨBa��OH��2��Һ��Ҳ���������ɣ����а�ɫ������������
��������ʵ��ش��������⣺
��1����Һ��һ�������ڵ������� ��
��2��һ�����ڵ������� ��
��3���жϳ����ҳɷֵķ����� ��
��MnO4-,Ag+,Ba2+,Al3+ ��CO32-,AlO2-
��ȡ��ɫ�����Ҽ���ϡ���ᡣ����ȫ�ܽ���ΪBaCO3������ȫ���ܽ���ΪBaSO4���������ܽ���ΪBaCO3��BaSO4�Ļ���
����:��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
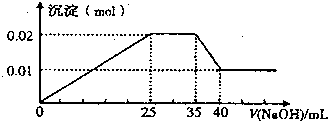
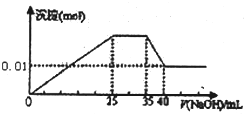 ��һδ֪����ɫ��Һ��ֻ���ܺ������������е������֣�H+��NH+4��K+��Mg2+��Cu2+��Al3+��NO-3��CO2-3��SO2-4����ȡ����100mL��Һ��������ʵ��
��һδ֪����ɫ��Һ��ֻ���ܺ������������е������֣�H+��NH+4��K+��Mg2+��Cu2+��Al3+��NO-3��CO2-3��SO2-4����ȡ����100mL��Һ��������ʵ���ٵ�һ�ݼ�����AgNO3��Һ���а�ɫ����������
�ڵڶ��ݼ�����BaCl2��Һ���а�ɫ������������ϴ�ӡ������������Ϊ6.99g��
�۵�������εμ�NaOH��Һ����ó�����NaOH��Һ�������ϵ��ͼ��
��������ʵ�飬�����Ʋⲻ��ȷ���ǣ�������
| A��ԭ��Һһ��������H+��Cu2+��CO2-3 | B������ȷ��ԭ��Һ�Ƿ���K+��NO-3 | C��ԭ��Һȷ����Mg2+��Al3+��NH+4����n��Mg2+����n��Al3+����n��NH+4��=1��1��2 | D��ʵ�����ӵ�NaOH��Ũ��Ϊ2 mol?L-1 |
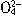 ��N
��N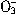 ��I-��S2-��H+��K+�������е����ֻ���֡���������ʵ�飺��ȡ������Һ����������������ų�������ȡԭ��Һ����Na2SO3��Һ��Ҳ������ų������а�ɫ�������ɣ��ټ������������ȫ��ʧ������ȡԭ��Һ����AgNO3��Һ�������ɡ��ɴ��жϣ���1��ԭ��Һ��һ�����е�������__________________����2��һ��������������________________����3�����ܺ��е�������______________��
��I-��S2-��H+��K+�������е����ֻ���֡���������ʵ�飺��ȡ������Һ����������������ų�������ȡԭ��Һ����Na2SO3��Һ��Ҳ������ų������а�ɫ�������ɣ��ټ������������ȫ��ʧ������ȡԭ��Һ����AgNO3��Һ�������ɡ��ɴ��жϣ���1��ԭ��Һ��һ�����е�������__________________����2��һ��������������________________����3�����ܺ��е�������______________��