��Ŀ����
������500mL��0.1mol/L��̼������Һ���ش��������⣺
��1��ͨ�������֪��Ӧ��������ƽ��ȡ
��2���������ҩƷλ�÷ŵߵ����������ʱδ���ձ���5g��ʹ�����룩����ƽƽ��ʱ��ʵ�ʳƵõ�̼���ƾ����������
��3�����в���ʹ������ҺŨ��ƫ�ߵ���
�ٳ�ȡ14.3gNa2CO3��������
�ڳ����������������
��������ƿת��ʱ��������Һ�彦��
��̼�����к��в���������
��δϴ���ܽ�Na2CO3���ձ�
����ʱ���ӿ̶���
��С�ձ�ϴ����δ���T����������
��1��ͨ�������֪��Ӧ��������ƽ��ȡ
14.3
14.3
g Na2CO3?10H2O����2���������ҩƷλ�÷ŵߵ����������ʱδ���ձ���5g��ʹ�����룩����ƽƽ��ʱ��ʵ�ʳƵõ�̼���ƾ����������
5.7
5.7
g����3�����в���ʹ������ҺŨ��ƫ�ߵ���
�٢ڢ�
�٢ڢ�
���ٳ�ȡ14.3gNa2CO3��������
�ڳ����������������
��������ƿת��ʱ��������Һ�彦��
��̼�����к��в���������
��δϴ���ܽ�Na2CO3���ձ�
����ʱ���ӿ̶���
��С�ձ�ϴ����δ���T����������
��������1������500mL��0.1mol/L��̼������Һ�к��е�̼���Ƶ����ʵ����������Ҫ̼���Ƶ�������
��2��������ƽ�ij���ԭ��������Ʒ������ߵ���ʵ�ʳ�����̼���Ƶ�������
��3������c=
�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��
��2��������ƽ�ij���ԭ��������Ʒ������ߵ���ʵ�ʳ�����̼���Ƶ�������
��3������c=
| n |
| V |
����⣺��1��500mL��0.1mol/L��̼������Һ�к��е�̼���Ƶ����ʵ���Ϊ��0.1mol/L��0.5L=0.05mol����ҪNa2CO3?10H2O����Ϊ��286g/mol��0.05mol=14.3g��
�ʴ�Ϊ��14.3��
��2����ƽ����̼���ƾ���ʱ������ϵΪ��m�����룩+m�����룩=m��ҩƷ��������14.3g̼���ƾ��壬��Ҫ����10g������4.3g����������̼���Ƶߵ���������ϵΪ��m��Na2CO3?10H2O��+4.3g=10g��������Na2CO3?10H2O������Ϊ��m��Na2CO3?10H2O��=10g-4.3g=5.7g��
�ʴ�Ϊ��5.7��
��3���ٳ�ȡ14.3gNa2CO3�������ƣ�������Һ�����ʵ����ʵ���ƫ����c=
�ɵã����Ƶ���ҺŨ��ƫ�ߣ��ʢ���ȷ��
�ڳ���������������룬���³�����ҩƷ����ƫ�����Ƶ���Һ�����ʵ����ʵ���ƫ����c=
�ɵã����Ƶ���ҺŨ��ƫ�ߣ��ʢ���ȷ��
��������ƿת��ʱ��������Һ�彦�����������Ƶ���Һ�����ʵ����ʵ���ƫ�ͣ�����c=
�ɵã���Һ��Ũ��ƫ�ͣ��ʢ۴���
��̼�����к��в��������ʣ��������ʵ����ʵ���ƫС������c=
�ɵã���Һ��Ũ��ƫ�ͣ��ʢܴ���
��δϴ���ܽ�Na2CO3���ձ����������ʵ����ʵ���ƫС������c=
�ɵã����Ƶ���ҺŨ��ƫ�ͣ��ʢݴ���
����ʱ���ӿ̶��ߣ����¼��������ˮ���ƫС������c=
�ɵã����Ƶ���ҺŨ��ƫ�ߣ��ʢ���ȷ��
��С�ձ�ϴ����δ���T�����������������õ����ձ�����������Բ�Ӱ�����ƽ�����ʢߴ���
��ѡ�٢ڢޣ�
�ʴ�Ϊ��14.3��
��2����ƽ����̼���ƾ���ʱ������ϵΪ��m�����룩+m�����룩=m��ҩƷ��������14.3g̼���ƾ��壬��Ҫ����10g������4.3g����������̼���Ƶߵ���������ϵΪ��m��Na2CO3?10H2O��+4.3g=10g��������Na2CO3?10H2O������Ϊ��m��Na2CO3?10H2O��=10g-4.3g=5.7g��
�ʴ�Ϊ��5.7��
��3���ٳ�ȡ14.3gNa2CO3�������ƣ�������Һ�����ʵ����ʵ���ƫ����c=
| n |
| V |
�ڳ���������������룬���³�����ҩƷ����ƫ�����Ƶ���Һ�����ʵ����ʵ���ƫ����c=
| n |
| V |
��������ƿת��ʱ��������Һ�彦�����������Ƶ���Һ�����ʵ����ʵ���ƫ�ͣ�����c=
| n |
| V |
��̼�����к��в��������ʣ��������ʵ����ʵ���ƫС������c=
| n |
| V |
��δϴ���ܽ�Na2CO3���ձ����������ʵ����ʵ���ƫС������c=
| n |
| V |
����ʱ���ӿ̶��ߣ����¼��������ˮ���ƫС������c=
| n |
| V |
��С�ձ�ϴ����δ���T�����������������õ����ձ�����������Բ�Ӱ�����ƽ�����ʢߴ���
��ѡ�٢ڢޣ�
���������⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ��������������������е��Ѷȵ����⣬���������ǿ�������߿��������������У�ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������������ѵ�������������ע�����շ������ķ����ͼ��ɣ�

��ϰ��ϵ�д�
�����Ŀ
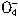 +5Cu2S+44H+=10Cu2++5SO2+8Mn2++22H2O
+5Cu2S+44H+=10Cu2++5SO2+8Mn2++22H2O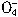 +5CuS+28H+=5Cu2++5SO2+6Mn2++14H2O
+5CuS+28H+=5Cu2++5SO2+6Mn2++14H2O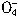 +______Fe2++______H+=______Mn2++_______Fe3++_______H2O
+______Fe2++______H+=______Mn2++_______Fe3++_______H2O