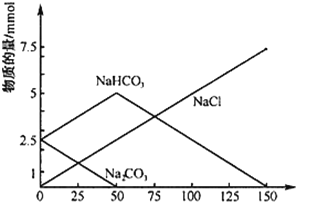
ЬтФПФкШн
ЁОЬтФПЁПвбжЊWЁЂYЁЂZЁЂTОљЮЊЖЬжмЦкдЊЫи,ЧвдзгАыОЖвРДЮдіДѓЁЃЧыЬюПе:
ЃЈ1ЃЉWЁЂZЪЧаЮГЩЛЏКЯЮяжжРрзюЖрЕФСНжждЊЫи,аДГіZдзгЕФКЫЭтЕчзгХХВМЭМ____;
ЃЈ2ЃЉЛЏКЯЮяYW3ЕФЫЎШмвКФмЪЙЗгЬЊБфКь,гУЗНГЬЪНБэЪОЗгЬЊБфКьЕФдвђ_________;
ЃЈ3ЃЉдЊЫиTЕФдзгжаЕчзгЙВеМОнСЫ7ИіЙьЕР,дђTдкдЊЫижмЦкБэ____жмЦк____зхЁЃTЕФЛЏКЯЮяTYШлШкЪБВЛЕМЕч,ГЃгУзїЩАТжМАФЭИпЮТВФСЯ,гЩДЫЭЦжЊ,ЫќЪєгк_____.
aРызгОЇЬх b.дзгОЇЬх c.ЗжзгОЇЬх d.ЮоЗЈХаЖЯЖЯ
ЃЈ4ЃЉYW3Ъєгк______(ЬюЁАМЋадЁБЁЂЁАЗЧМЋадЁБ)ЗжзгЦфжаYЕФЛЏКЯМлЮЊ______.
ЁОД№АИЁП ![]() NH3ЁЄH2ONH4++OH- ЕкШ§ ЕкЂѓA b МЋад -3
NH3ЁЄH2ONH4++OH- ЕкШ§ ЕкЂѓA b МЋад -3
ЁОНтЮіЁПгЩЬтвтПЩжЊWЮЊHЁЂZЮЊCЃЌCЕФЕчзгХХВМЭМЮЊЃК![]() ЁЃ
ЁЃ
(2)гЩЬтвтПЩжЊYW3ЮЊNH3ЃЌгЩгкNH3ЃЋH2O![]() NH3ЁЄH2O
NH3ЁЄH2O![]() NH4+ЃЋOHЃЫљвдNH3ЕФЫЎШмвКЯдМюадЁЃ
NH4+ЃЋOHЃЫљвдNH3ЕФЫЎШмвКЯдМюадЁЃ
(3)гЩКѓАыОфЁАTЕФЛЏКЯЮяTYЁЁЁБПЩжЊTYЮЊдзгОЇЬхЃЌYЮЊNЃЌдђTЮЊAlЃЌЧвAlЕФЕчзгХХВМЪНЮЊЃК1s22s22p63s23p1еМОнСЫ7ИіЙьЕРЁЃ
(4)YW3ЮЊNH3ЃЌПеМфЙЙаЭЮЊШ§НЧзЖаЮЃЌЙЪЮЊМЋадЗжзгЃЌHЮЊЃЋ1МлЃЌNЮЊЃ3МлЁЃ

ЁОЬтФПЁПЮяжЪAЁЋEОљКЌЭЌжждЊЫиЃЌЖМЪЧжабЇЛЏбЇжаГЃМћЕФЮяжЪЃЌCЮЊРЖЩЋВЛШмгкЫЎЕФЙЬЬхЃЌЫќУЧПЩЗЂЩњШчЯТЭМЫљБэЪОЕФЗДгІ(Г§AЁЋEЭтЕФЦфЫћЮяжЪвбТдШЅ)ЃК

(1)аДГіЯргІЮяжЪЕФРрБ№ЃК
ЮяжЪ | B | C | D |
РрБ№ | ___________ | ______________ | ______________ |
(2)дквдЩЯЗДгІжа(гУађКХЬюПе)ЃК
ЪєгкбѕЛЏЛЙдЗДгІЕФЪЧ________ЃЛЪєгкИДЗжНтЗДгІЕФЪЧ________ЁЃ
(3)аДГіЗДгІЂлКЭЂпЕФРызгЗНГЬЪНЃК_______________________ЁЃ