��Ŀ����
���¸����и����Ĺ�ϵ�����á�������������������ʾ��
��1��pH �� 3�Ĵ����pH��11������������Һ�������ϣ����Һ��c(Na��) c(CH3COO��)
��2�������ʵ���Ũ�ȡ����������ʹ����ƻ�ϣ����Һ�и�����Ũ�ȵĴ�С��ϵΪ
��3�������£���NaOH��Һ�е�c(OH��)��NH4Cl��Һ�е�c(H��)��ͬ���ֽ�NaOH��NH4Cl����Һ�ֱ�ϡ��10����ϡ�ͺ�NaOH��NH4Cl��Һ��pH�ֱ���pH1��pH2��ʾ����pH1�� pH2 14
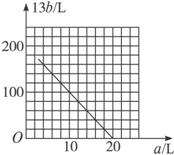
��4������pH��ȡ��������Ba(OH)2��NaOH��NH3��H2O������Һ�������Ƿֱ���V1 L��V2 L��V3 L��Ũ�ȵ������ϣ���Ϻ���Һ�������ԣ���V1 ��V2 ��V3�Ĵ�С��ϵ
��1��c(Na��)�� c(CH3COO��)��2�� c(Cl��)��c(Na��)��c(H��)��c(CH3COO��)��c(OH��)
��3��pH1�� pH2 �� 14 ��4��V1��V2��V3�Ĵ�С��ϵ V3�� V1 ��V2
����

��ϰ��ϵ�д�
�����Ŀ