��Ŀ����
����������¿�������������������4FeS2��11O2��8SO2��2Fe2O3���������N2��O2�ĺ����ֱ�Ϊ
0.800��0.200(����������������庬���������������ʾ)����������и��⣺
��1��1.00molFeS2��ȫ��������Ҫ���������(��״��)Ϊ______________L
��2��55L����������FeS2��ȫ��Ӧ���������(ͬ��ͬѹ)��Ϊ______________L
��3���ÿ�������FeS2���������������У�O2�ĺ���Ϊ0.0800��SO2�ĺ���Ϊ_______________
��4����FeS2������������������Ϊ100L������O2ΪaL��SO2ΪbL��
�� д��a��b�Ĺ�ϵʽ_______________
�� ��ͼ�л���a��b�Ĺ�ϵ����(FeS2����ʱ����������20%)
˵����Ϊ������ͼ����������13b��ʾ
0.800��0.200(����������������庬���������������ʾ)����������и��⣺
��1��1.00molFeS2��ȫ��������Ҫ���������(��״��)Ϊ______________L
��2��55L����������FeS2��ȫ��Ӧ���������(ͬ��ͬѹ)��Ϊ______________L
��3���ÿ�������FeS2���������������У�O2�ĺ���Ϊ0.0800��SO2�ĺ���Ϊ_______________
��4����FeS2������������������Ϊ100L������O2ΪaL��SO2ΪbL��
�� д��a��b�Ĺ�ϵʽ_______________
�� ��ͼ�л���a��b�Ĺ�ϵ����(FeS2����ʱ����������20%)
˵����Ϊ������ͼ����������13b��ʾ

��1��308
��2��52
��3����SO2ΪX(�������)������
����4��(11��X/8��0.0800)��1��0.0800��X
����X��0.0923
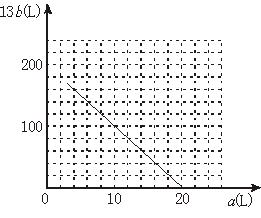
��4��13b��200��10a����ͼ���ԡ�
��2��52
��3����SO2ΪX(�������)������
����4��(11��X/8��0.0800)��1��0.0800��X
����X��0.0923
��4��13b��200��10a����ͼ���ԡ�

��ϰ��ϵ�д�
�����Ŀ