��Ŀ����
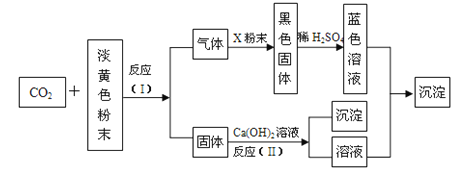
����Ŀ�����ۡ�8.12����ը�¹��У���ը��������軯��������й©����ͨ�����������������Һ���������Լ�����Ⱦ��ij��ѧ��ȤС����ʵ�����Ʊ���������ƣ���̽����������Ƶ����ʼ��軯�Ʒ�ˮ�Ĵ�����
��ʵ��һ��ʵ����ͨ������ͼ��a����ʾװ���Ʊ�Na2S2O3��5H2O
��֪��Na2S2O3��������Һ�в����ȶ����ڣ��й����ʵ��ܽ��������ͼ��b����ʾ��
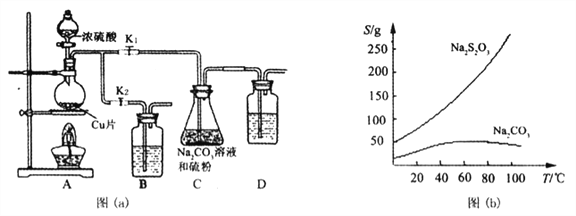
����1����ͼ���Ӻ�װ�ú��A��Cװ�������ԵIJ�����__________��
����2������ҩƷ����K1���ر�K2�����ȡ�װ��B��D�е�ҩƷ��ѡ������__________�����ţ�
A. NaOH��Һ B.ŨH2SO4 C.����KMnO4��Һ D.����NaHCO3��Һ
����3��C�л��Һ��������������Ӧһ��ʱ�����۵������١���C����Һ��pH��7��10ʱ����K2���ر�K1��ֹͣ���ȣ�C����ҺҪ����pH��ԭ����__________��
����4���Ƚ�C�еĻ��Һ���ˣ��ٽ���Һ��������Ũ�������ȹ��ˣ������Һ__________�õ���Ʒ��
��ʵ�����Na2 S2O3�����ʼ���ˮ������
��1����������������ˮ�еμ�����Na2 S2O3��Һ����ˮ��ɫ��dz����鷴Ӧ����Һ�к����������д���÷�Ӧ�����ӷ���ʽ__________��
��2���軯������������Ƶķ�ӦΪ��NaCN + Na2S2O3��NaSCN + Na2SO3����֪��NaSCN��SΪ-2�ۣ�������lmolNaCN��̼ԭ��ʧȥ���ӵ����ʵ���Ϊ__________��
��3����ˮ�е�CN��Ҳ���ڴ�������������NaClO������CNO�����������������¼�����NaClO��CNO�����������ֶԻ�������Ⱦ�����塣���һ����Ӧ�����ӷ���ʽΪ��
__________��
����������
���𰸡� �ر�K2,��K1,��D�м�ˮ����û����ĩ�ˣ��رշ�Һ©��������ƿ�����ܿڲ����������ݣ�ֹͣ���Ȳ�������ˮ���� ACD ����ͨSO2��ʹ��ҺPH��һ�����Ͷ�Na2S2O3��������Һ�в����ȶ����ڡ� ��ȴ�ᾧ�����ˡ�ϴ�ӡ���� S2O32-+ 4Cl2 +5H2O��SO42-+10H++8Cl- 2 mol 2CNO- +2H++3ClO-��N2��+2CO2��+3C1-+H2O
�������������������ʵ��һ����1�����ݼ��װ�õ������Եij��÷�����װ��B��D��������β������������Na2S2O3��������Һ�в����ȶ��������ش�C����ҺҪ����pH��ԭ����Na2S2O3���ܽ�����¶����߶������������4����ʵ�������1����ˮ��ɫ��dz����鷴Ӧ����Һ�к����������˵��������S2O32-����Ϊ��������ӣ���2��������NaCN��NaSCN��CԪ�ػ��ϼ۷�������3�������������¼�����NaClO��CNO�����������ֶԻ�������Ⱦ�������Ƕ�����̼�͵�����
��������ʵ��һ����1�����ݼ��װ�õ������Եķ��������A��Cװ�������ԵIJ����ǹر�K2,��K1����D�м�ˮ����û����ĩ�ˣ��رշ�Һ©��������ƿ�����ܿڲ����������ݣ�ֹͣ���Ȳ�������ˮ����װ��B��D��������β�����������������ܱ�������Һ����������Һ���գ�ѡ��NaOH��Һ������KMnO4��Һ������NaHCO3��Һ�� Na2S2O3��������Һ�в����ȶ�����������C����ҺҪ����pH�ڼ��Է�Χ���� Na2S2O3���ܽ�����¶����߶�����������Һ��ȴ�ᾧ�����ˡ�ϴ�ӡ���ɵõ�Na2S2O3��5H2O��
��ʵ�������1����ˮ��ɫ��dz����鷴Ӧ����Һ�к����������˵��������S2O32-����Ϊ�������������Ӧ���ӷ���ʽΪS2O32-+ 4Cl2 +5H2O��SO42-+10H++8Cl-����2��NaCN��C Ԫ�ػ��ϼ�Ϊ+2��NaSCN��CԪ�ػ��ϼ�Ϊ+4������,lmolNaCN��̼ԭ��ʧȥ���ӵ����ʵ���Ϊ2mol����3�������������¼�����NaClO��CNO�����������ֶԻ�������Ⱦ�������Ƕ�����̼�͵��������ӷ���ʽΪ 2CNO- +2H++3ClO-��N2��+2CO2��+3C1-+H2O��