��Ŀ����
����Ŀ��NaClO2��һ����Ҫ��ɱ����������Ҳ������Ư��֯��ȣ���֪��NaClO2������Һ���¶ȵ���38��ʱ��������NaClO2��3H2O���¶ȸ���38��ʱ��������NaClO2���¶ȸ���60��ʱNaClO2�ֽ�����NaClO3��NaCl,��һ�������������¡�
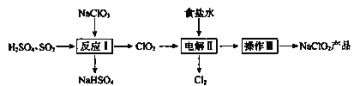
��ش��������⣺
(1)NaClO2��ClԪ�صĻ��ϼ�Ϊ____________��
(2)��ӦI��
��SO2��___________(�����������ԭ����)��
��д����ӦI�Ļ�ѧ����ʽ��__________________________��
(3)���II��
������ʳ��ˮ�ɴ���ˮ���ƶ��ɣ�����ʱ��Ϊ��ȥ����ˮ�е�Mg2+��Ca2+����Ҫ������Լ��ֱ�Ϊ_______��______��
�ڵ������б�������������________��
�ۿ���NaOH��Һ���յ�������Cl2����Ӧ�����ӷ���ʽ��______________��
(4)����III��
��Һ����NaClO2�IJ������裺�ټ����������ڳ��ȹ��ˣ���ϴ�ӣ��ܸ���õ���Ʒ�����ȹ���Ӧ���Ƶ��¶ȷ�Χ��___________��
���𰸡�+3 ��ԭ�� 2NaClO3+SO2+H2SO4=2ClO2+2NaHSO4 ����������Һ ̼������Һ NaCl��Cl- Cl2+2OH- =Cl-+ClO-+H2O 38��-60��
��������
�ɻ�ѧ�������̿�֪����H2SO4�ữ�����£�NaClO3��SO2����������ԭ��Ӧ����ClO2��ΪNaHSO4����Ӧ��NaClO3����������SO2�ǻ�ԭ�������װ���У�ClO2�������õ��ӷ�����ԭ��Ӧ����ClO2-��Cl-������ʧ���ӷ���������Ӧ����Cl2�� NaClO2��Һ�������ᾧ�����ȹ��ˡ�ϴ�ӡ�����õ���Ʒ��
��1��NaClO2��Na��+1�ۣ�O�ǣ�2�ۣ�����ݻ������������۴�����Ϊ0��֪Cl�Ļ��ϼ���+3�ۣ��ʴ�Ϊ��+3��
��2����H2SO4�ữ�����£�NaClO3��SO2����������ԭ��Ӧ����ClO2��ΪNaHSO4����Ӧ��NaClO3����������SO2�ǻ�ԭ������Ӧ�Ļ�ѧ����ʽΪ2NaClO3+SO2+H2SO4=2ClO2+2NaHSO4���ʴ�Ϊ����ԭ����2NaClO3+SO2+H2SO4=2ClO2+2NaHSO4��
��3�����������ӵ����ʣ���ȥ����ˮ�е�Mg2+��Ca2+Ӧѡ�ù�����̼������Һ��ȥ�����ӣ�ѡ������������Һ��ȥþ���ӣ��ʴ�Ϊ������������Һ��̼������Һ��
�����װ���У�ClO2�������õ��ӷ�����ԭ��Ӧ����ClO2-��Cl-������ʧ���ӷ���������Ӧ����Cl2����������б��������������Ȼ��ƣ��ʴ�Ϊ��NaCl��Cl-��
������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ����Ӧ�����ӷ���ʽΪCl2+2OH����Cl��+ClO��+H2O���ʴ�Ϊ��Cl2+2OH����Cl��+ClO��+H2O��
��4���������Ϣ��֪��NaClO2������Һ���¶ȵ���38��ʱ��������NaClO2��3H2O���¶ȸ���38��ʱ��������NaClO2���¶ȸ���60��ʱNaClO2�ֽ����������ƺ��Ȼ��ƣ���˳��ȹ���Ӧ���Ƶ��¶ȷ�Χ��38����60�����ʴ�Ϊ��38����60����

����Ŀ����ҽ�����������鷨�����Ƿ���˪��As2O3���ж�,�漰�ķ�Ӧ���£�
��: 6Zn+As2O3+12HCl=6ZnCl2+2AsH3(����) ��+3H2O
��: 2AsH3=2As(��ɫ�龵)+3H2
��1��д����Ļ�̬ԭ�Ӽ۵����Ų�ʽ__________
��2������Ŀռ�ṹΪ_________����������ԭ���ӻ���ʽΪ________
��3������ͬ��ͬϵ����������������±���
NH3 | PH3 | AsH3 | SbH3 | |
�۵�/�� | -77.8 | -133.5 | -116.3 | -88 |
�е�/�� | -34.5 | -87.5 | -62.4 | -18.4 |
��PH3![]() AsH3
AsH3![]() SbH3���۷е��������ߵ�ԭ����______________________��NH3���������ԭ����__________________________________.
SbH3���۷е��������ߵ�ԭ����______________________��NH3���������ԭ����__________________________________.
��4����һ����������I(As)____ I(Se)
��5������������BN�����������ĥ���������ϣ��侧���ṹ����ʯ���ƣ���ͼ��ʾ��
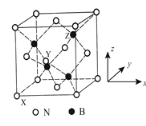
��������������Ҫ�أ�ԭ�������������ʾ�����ڲ���ԭ�ӵ����λ�á���ͼb��ʾ������ԭ���������XΪ��0��0��0����Yԭ�ӵ��������Ϊ��1/2��0��1/2������Zԭ�ӵ��������Ϊ________���������������������Ĵ�С����״����֪������������ܶ�Ϊd gcm��3�������ӵ�����ֵΪNA��������a��________nm�����г�����ʽ���ɣ�