��Ŀ����
��֪X��Y��Z��L����Ԫ������ɵ����ʵĻ���Ԫ����ԭ�������������ش��������⣺
��1��L��Ԫ�ط���Ϊ________ ��YԪ��ԭ�Ӻ�������У�δ�ɶԵ�������ɶԵ�����֮��Ϊ______������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����____________________����Ԫ�ط��ű�ʾ����
��2��Z��X��Ԫ�ذ�ԭ����Ŀ��l��3�ɹ��ɷ���A��A�ĵ���ʽΪ________��B����Ҳ��Z��X��Ԫ����ɣ���Ϊ���ͷɴ��Ļ��ȼ�ϣ���������һ��Һ̬�������֪�û��������Է�������Ϊ32������XԪ�ص���������Ϊ12��5 %���Ҹ÷��ӽṹ��ֻ�е�������B�ĽṹʽΪ____________����64 g B������Һ̬˫��ˮǡ����ȫ��Ӧ�������������ֲ���Ⱦ��������̬���ʣ����ų�3000 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
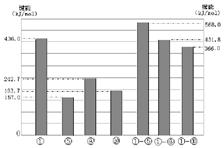
��3������Se��������������Ԫ�أ���Ԫ��Lͬһ���壬�����2 ~5����Ԫ�ص��ʷֱ���H2��Ӧ����l mol��̬�⻯������������������£������ܱ�ʾ����1 mol����������������������__________��ѡ����ĸ��ţ���
a����99��7 kJ b����29��7 kJ c��+20��6 kJ d��+241��8 kJ
��1��L��Ԫ�ط���Ϊ________ ��YԪ��ԭ�Ӻ�������У�δ�ɶԵ�������ɶԵ�����֮��Ϊ______������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����____________________����Ԫ�ط��ű�ʾ����
��2��Z��X��Ԫ�ذ�ԭ����Ŀ��l��3�ɹ��ɷ���A��A�ĵ���ʽΪ________��B����Ҳ��Z��X��Ԫ����ɣ���Ϊ���ͷɴ��Ļ��ȼ�ϣ���������һ��Һ̬�������֪�û��������Է�������Ϊ32������XԪ�ص���������Ϊ12��5 %���Ҹ÷��ӽṹ��ֻ�е�������B�ĽṹʽΪ____________����64 g B������Һ̬˫��ˮǡ����ȫ��Ӧ�������������ֲ���Ⱦ��������̬���ʣ����ų�3000 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��3������Se��������������Ԫ�أ���Ԫ��Lͬһ���壬�����2 ~5����Ԫ�ص��ʷֱ���H2��Ӧ����l mol��̬�⻯������������������£������ܱ�ʾ����1 mol����������������������__________��ѡ����ĸ��ţ���
a����99��7 kJ b����29��7 kJ c��+20��6 kJ d��+241��8 kJ
��1��O��1��2�� C��N��O��H ��1��1��3�֣�
��2��
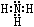 ��1�֣���
��1�֣��� 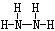 ��1�֣���N2H4(l)+2H2O2(l)��N2(g)+4H2O(g)+1500 kJ��2�֣�
��1�֣���N2H4(l)+2H2O2(l)��N2(g)+4H2O(g)+1500 kJ��2�֣���3��b ��1�֣�
�����������1��Y��C Cԭ�ӵĺ�������Ų�ʽ1s22s22p2 ��p�����2��δ�ɶԵ��ӣ��ɶԵ��Ӷ���4��
δ�ɶԵ�������ɶԵ�����֮��Ϊ1:2�� X��H Y:C Z:N L:O ԭ�Ӱ뾶��C��N��O��H ��
��2��Z��N X��H AΪNH3 ����֪�û��������Է�������Ϊ32������XԪ�ص���������Ϊ12��5 %��X��32*0��125/1=4 Z:(32-4)/14=2 B�ķ���ʽ��N2H4 B�Ľṹʽ��
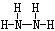 ��64���൱��2mol N2H4�ų�����Ϊ3000 kJ��1mol N2H4�ų�����Ϊ1500 kJ���Ȼ�ѧ����ʽ��N2H4(l)+2H2O2(l)��N2(g)+4H2O(g)+1500 kJ
��64���൱��2mol N2H4�ų�����Ϊ3000 kJ��1mol N2H4�ų�����Ϊ1500 kJ���Ȼ�ѧ����ʽ��N2H4(l)+2H2O2(l)��N2(g)+4H2O(g)+1500 kJ
��ϰ��ϵ�д�
�����Ŀ
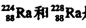 ����������ͬ��������ͬ��ͬ�ֺ���
����������ͬ��������ͬ��ͬ�ֺ���
 2A(g) ��H =��92.4 kJ?mol�D1
2A(g) ��H =��92.4 kJ?mol�D1