��Ŀ����
13����ͼ��ʾ��ijͬѧ�����һ��ȼ�ϵ�ز�̽���ȼҵԭ���ʹ�ͭ�ľ���ԭ����������װ����XΪ�����ӽ���Ĥ���밴Ҫ��ش�������⣺��1������ȼ�ϵ�ظ�����Ӧʽ��CH4+10OH--8e-=CO32-+7H2O��
��2��ʯī��C�����ĵ缫��ӦʽΪ2Cl--2e-=Cl2����
��3�����ڱ�״���£���2.24L�����μӷ�Ӧ������װ�������������ɵ��������Ϊ4.48L����װ������������ͭ������Ϊ12.8g��
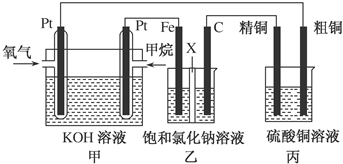
���� ȼ�ϵ���У�Ͷ��ȼ�ϵĵ缫�Ǹ�����Ͷ���������ĵ缫��������
��1��ȼ�ϵ���У�������Ͷ��ȼ�ϣ�������ʧ���ӷ���������Ӧ��
��2������Ͷ���������ĵ缫��������������װ����ʯī��������������������ʧ���ӷ���������Ӧ��
��3�����ݴ��������ת�Ƶ�������ȼ������缫�������������������װ��������ͭ��������
��� �⣺ȼ�ϵ���У�Ͷ��ȼ�ϵĵ缫�Ǹ�����Ͷ���������ĵ缫��������
��1��ȼ�ϵ���У�������Ͷ��ȼ������Ͷ�ż���ĵ缫�Ǹ�����������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��
CH4+10OH--8e-=CO32-+7H2O��
�ʴ�Ϊ��CH4+10OH--8e-=CO32-+7H2O��
��2������Ͷ���������ĵ缫��������������װ����ʯī�缫��������������������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��2Cl--2e-=Cl2����
�ʴ�Ϊ��2Cl--2e-=Cl2����
��3�����������ת�Ƶ�������ȣ����ڱ�״���£���2.24L�����μӷ�Ӧ����ת�Ƶ��ӵ����ʵ���=$\frac{2.24L}{22.4L/mol}$����װ�������缫�������ӷŵ��������������������������ΪxL����װ��������������ͭ��������ͭ������Ϊyg��
2H++2e-=H2��
2mol 22.4L
0.4mol xL
x=4.48
2Cu 2++2e-=Cu
2mol 64g
0.4mol yg
y=12.8
�ʴ�Ϊ��4.48��12.8��
���� ���⿼����ԭ��غ͵���ԭ�������ʵ������йؼ��㣬�ѶȲ�����Ҫ�绯ѧװ�õ��жϺ�ԭ���ķ�����

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�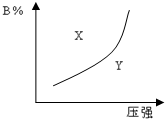 mA��g��+nB��g��?pC��g��������ӦΪ���ȷ�Ӧ���Ŀ��淴Ӧ���ں��������£�ƽ��ʱB�ڻ�����еĺ�����B%����ѹǿ�Ĺ�ϵ��ͼ��ʵ����ʾ���й�������ȷ���ǣ�������
mA��g��+nB��g��?pC��g��������ӦΪ���ȷ�Ӧ���Ŀ��淴Ӧ���ں��������£�ƽ��ʱB�ڻ�����еĺ�����B%����ѹǿ�Ĺ�ϵ��ͼ��ʵ����ʾ���й�������ȷ���ǣ�������| A�� | m+n��p | B�� | x������ʹ�ϵΪv����v�� | ||
| C�� | n��p | D�� | x���y�����ﷴӦ���ʿ� |
| ��ѧ�� | H-H | Cl-Cl | H-Cl |
| ���ܨM��kJ•mol-1�� | 436 | 243 | 431 |
| A�� | 0.5 H2��g��+0.5 Cl2��g���THCl��g����H=-91.5 kJ•mol-1 | |
| B�� | H2��g��+Cl2��g���T2HCl��g����H=-183 kJ•mol-1 | |
| C�� | 0.5 H2��g��+0.5 Cl2��g���THCl��g����H=+91.5 kJ•mol-1 | |
| D�� | 2HCl��g���TH2��g��+Cl2��g����H=+183 kJ•mol-1 |
 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������100mL 1mol•L-1��ϡ���ᣮ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ����ش��������⣺
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������100mL 1mol•L-1��ϡ���ᣮ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ����ش��������⣺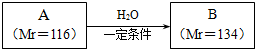
 ��
�� ��
�� ��
��