��Ŀ����
13��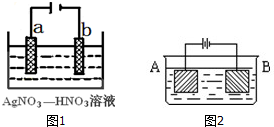 ����ұ���ʹ������漰�绯ѧ��Ӧ��
����ұ���ʹ������漰�绯ѧ��Ӧ����1��ұ����ұ���ᴿ������Ӧ����ʱû�в��õ���ⷨ����a
a��Feb��Na c��Cu d��Al
��2����ͼ1Ϊ��⾫������ʾ��ͼ��a����a��b����Ϊ�������ʵĴ������������У���b������������ɫ�������ɣ��ڿ����б����ɫ������������ĵ缫��ӦʽΪNO3-+e-+2H+=NO2��+H2O��
��3���������������þú�����׳��ֺڰߣ�Ag2S����Ϊ�����ڰߣ����������������������ʳ��ˮ�в������Ӵ���Ag2Sת��ΪAg��ʳ��ˮ������Ϊ���������Һ���γ�ԭ��أ�
��4����ͼ2��װ�ý��е��ʵ�飺A����ͭп�Ͻ�B��Ϊ��ͭ�������ΪCuSO4��Һ����������ͨ��һ��ʱ���A��ǡ��ȫ���ܽ⣬��ʱB����������7.68g����Һ��������0.03g����A�Ͻ���Cu��Znԭ�Ӹ�����Ϊ3��1��
���� ��1����ⷨ��ұ�����ý���K��Ca��Na��Mg��Al��һ���õ�����ڵ��Ȼ��Al�ǵ�����ڵ��������������Ƶã��Ȼ�ԭ����ұ���ϲ����õĽ���Zn��Fe��Sn��Pb��Cu�����û�ԭ���У�C��CO��H2�ȣ����ȷֽⷨ��Hg��Ag�ü��ȷֽ�������ķ����Ƶã��������뷨��Pt��Au����������ķ����Ƶã�
��2����⾫����ʱ����������������b������������ɫ�������ɣ���b�缫����������ӵõ������ɶ���������
��3��Ϊ������������ĺڰߣ�Ag2S�������������������������ʳ��ˮ�в������Ӵ�����װ�ù���ԭ��أ�ʳ��ˮ���������Һ���ٽ���Һ�ĵ����ԣ�
��4����װ���ǵ��أ������Ͻ���ʧ���ӷ���������Ӧ��������ͭ���ӵõ��ӷ�����ԭ��Ӧ���������������ӵ�������ͭ����������Һ�����ӵ�����Ϊ�ܽ�п��������ͨ����ͬ����ʱ����ͭ�������������������Լ����ܽ��п�����ʵ������ٸ���������ԭ��Ӧ�е�ʧ��������ȼ���Ͻ���ͭ�����ʵ������Ӷ�����ͭ��п��ԭ�Ӹ���֮�ȣ�
��� �⣺��1�����ý������õ�ⷨұ�����ơ������ǻ��ý��������õ������̬NaCl��Al2O3�ķ���ұ�������ᴿ��ͭʱҲ�õ�⾫��ͭ���ʴ�Ϊ��a��
��2����⾫��ʱ�����������������Դ�����a����b�缫��������������ԭ��Ӧ�������˺���ɫ������NO2���缫��Ӧ��NO3-+e-+2H+=NO2��+H2O��
�ʴ�Ϊ��a��NO3-+e-+2H+=NO2��+H2O��
��3����װ�ù���ԭ��أ��Ȼ�����Һ���������Һ���ٽ���Һ�ĵ���������
�ʴ�Ϊ�����������Һ���γ�ԭ��أ�
��4��B������������ͭ��B����������7.68g�������ʵ���=$\frac{7.68g}{64g/mol}$=0.12mol��
�������ܽ�пʱ������������ͭ��������Һ�������ӵ�����Ϊп��ͭ���������Һ��������0.03g����п��ͭ��������Ϊ0.03g��
��п�����ʵ���Ϊx��
Zn+Cu2+=Zn2++Cu ��������
1mol 1g
x 0.03g
x=0.03mol��
���Ͻ���п�����ʵ�����0.03mol��
����������ԭ��Ӧ�е�ʧ���������֪��������п��ͭʧȥ�ĵ���������������ͭ���ӵõ��ĵ��ӣ���ͭ�����ʵ���Ϊy��
0.03mol��2+2y=0.12mol��2
y=0.09mol��
����ͭ��п�����ʵ���֮��Ϊ0.09mol��0.03mol=3��1������ͭ��п��ԭ�Ӹ���֮����3��1���ʴ�Ϊ��3��1��
���� ���⿼���˽�����ұ��������������ԭ��Ӧ�����ԭ����֪ʶ�㣬���ݽ����Ļ�����ȷ��ұ�����������ӵķŵ�˳������������ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | 1.4 mol•L-1 | B�� | 1.0 mol•L-1 | C�� | 0.7 mol•L-1 | D�� | 0.5mol•L-1 |
| A�� | ������������һ�����ڷ�Ӧ�������� | |
| B�� | ͬ��ͬѹ�£�H2��g��+Cl2��g���T2HCl��g�� �ڹ��պ͵�ȼ�����µġ�H��ͬ | |
| C�� | ��֪N2��g��+3H2��g���T2 NH3��g������H=-92.4 kJ•mol-1������һ�������½�1molN2��3molH2����һ�ܱ������г�ַ�Ӧ�����ɷų�92.4kJ������ | |
| D�� | ���к��Ȳⶨ����IJ��������У�Ӧ��ʢ��ϡ�����С�ձ�����εμ���������ϡ��Һ |
| A�� | ���¶ȡ�ѹǿһ���������£������غ������ع�ͬ����һ����ѧ��Ӧ�ķ��� | |
| B�� | �¶ȡ�ѹǿһ��ʱ�����ȵ������ӵķ�Ӧһ�����Է����� | |
| C�� | ��Ӧ�ʱ��Ǿ�����Ӧ�Ƿ��Է����е�Ψһ���� | |
| D�� | ������ܽ�������ر��й� |
| A�� | ���ǿ��������Ƚ��Ļ�ѧ�������豸�����µ�ԭ�� | |
| B�� | ���ǿ������ô���ʹˮ������� | |
| C�� | ���ǿ��������Ƚ��Ļ�ѧ�������豸������Ȼ���в����ڵķ��� | |
| D�� | ��ѧ��ѧֻ��ͨ��ʵ����̽�����ʵ����� |
| A�� | pH=1 | B�� | pH��1 | C�� | pH��1 | D�� | ��ȷ�� |
| A�� | ���³�ѹ�£�32�������к���2NA��ԭ�� | |
| B�� | ��״���£�1L��ϩ��ȫȼ�պ���������̬����ķ�����Ϊ6NA/22.4 | |
| C�� | 78g Na2O2�����к��е���������ĿΪ2NA�� | |
| D�� | 1molNa2O2������CO2��Ӧת�Ƶĵ�������Ϊ2NA |
| A�� | �û�������ͭ | B�� | �������壬�û�������ͭ | ||
| C�� | ֻ������ɫ���� | D�� | �������壬������ɫ���� |