��Ŀ����
1 (13��) ��ͼ��ʾ�������ʼ�������ת����ϵ��
![]()

A��F�ֱ�Ϊ����ɫ���壬A��B��Ũ��Һ��Ӧ��������C��D��E�������ʣ�DΪ��ɫҺ�壬KΪ��ɫ�������ʣ����ڼ�����������C��Ũ��Һ��Ӧ����������Q��IΪ��̬˫ԭ�ӷ��ӡ���Ӧ�����в��������Ͳ������ȥ��
(1) ![]() I�ĵ���ʽ��____________________��
I�ĵ���ʽ��____________________��
(2) ![]() ��ӦA + B�Ļ�ѧ����ʽ��_________________________________________________��
��ӦA + B�Ļ�ѧ����ʽ��_________________________________________________��
(3) ![]() ��Q��E��2��1ͬʱͨ��Ʒ����Һ�У�������֮һ��I�����������Ϊ___________��ԭ���ǣ��û�ѧ����ʽ��ʾ��______________________________________________��
��Q��E��2��1ͬʱͨ��Ʒ����Һ�У�������֮һ��I�����������Ϊ___________��ԭ���ǣ��û�ѧ����ʽ��ʾ��______________________________________________��
(4) ![]() ����״���½�11.2 L Qͨ�뵽500 mL 1.5 mol /L ��G��Һ�У��뽫������Һ�����ʳɷּ����ʵ��������±����ɲ���������
����״���½�11.2 L Qͨ�뵽500 mL 1.5 mol /L ��G��Һ�У��뽫������Һ�����ʳɷּ����ʵ��������±����ɲ���������
���ʣ���ѧʽ�� | ���ʵ��� |
|
|
|
|
|
|
(1) 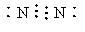 ��2�֣�
��2�֣�
(2)![]() ��3�֣�
��3�֣�
(3) Ʒ�첻��ɫ����û����������1�֣�
4SO2 + 2NO2 + 4H2O = N2 + 4H2SO4��3�֣�
(4)
Na2SO3 | 0.25 mol | ��4�֣� |
NaHSO3 | 0.25 mol |

 ��У����ϵ�д�
��У����ϵ�д���������������������Һ���кͷ�Ӧû�����Ե�����ijѧϰ��ȤС���ͬѧΪ��֤������������Һ����������ᷢ���˷�Ӧ�����кͷ�Ӧ����ЧӦ��������������漸��ʵ�鷽������ش��й����⡣
(1)����һ����ͼ1װ��ʵ��װ�ã�ͼ��С�Թ���ϸ�ߵ��ţ�ϸ�ߵ��϶�˩��ϸ��˿�ϡ���ʼʱʹ�Ҷ�U�ι����˺�īˮ��ƽ��ʵ�鿪ʼ�����²�ϸ��˿��ʹС�Թ���������ƿ������������Һ��ϣ���ʱ�۲쵽��������_________ ��ԭ����___________��
(2)����������С�������Ӧ��Һ�¶ȵı仯���жϷ�Ӧ�ķ������������������Һ��������ǰ�����¶ȵı仯����֤�������˻�ѧ��Ӧ����С��ͬѧ����ͬŨ�ȵ�����������Һ�������10 mL��ϣ����¶ȼƲ�����Ӧǰ���¶ȵı仯����õIJ����������±���
| ��� | ���� | �������� | ��t/�� |
| 1 | 0.1 mol��L��1 | 0.05 mol��L��1 | 3.5 |
| 2 | 0.1 mol��L��1 | 0.1 mol��L��1 | x |
| 3 | 0.2 mol��L��1 | 0.2 mol��L��1 | 14 |
(3)����������С�黹�������ͼ2ʾװ����֤������������Һȷʵ��ϡ���ᷢ���˷�Ӧ��������Ϊ��ϴ��ƿ�е��ܿ�������ð������˵���÷�Ӧ�ų��������Ӷ�֤�������˷�Ӧ��
��ʵ��ʱ����Һ©�����������ֵ�������Һ�岻����ԭ�������______________
�ڴ�ԭ���Ͻ�����ʵ����ƵIJ�����֮��Ϊ__________________________________��
�����ڴ�ʵ��װ�õĻ���������ķ���__________________________________��


 16��
16��