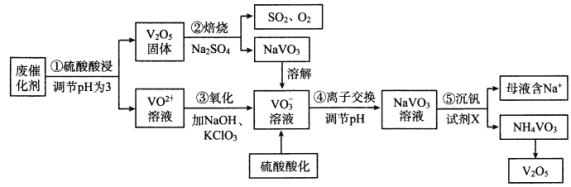
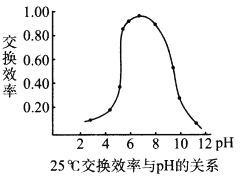
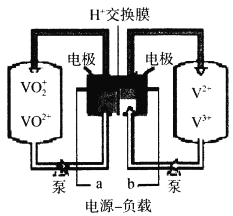
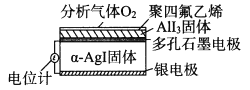
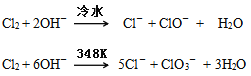��Ŀ����
����Ŀ������ijЩ�л���֮����ת�����Դ���̫���ܣ���ԭ����Ƭ��ϩ(NBD)����̫������ת����Ϊ�Ļ���(Q)����֪�� ��C7H8(l)(NBD)��9O2(g)=7CO2(g)��4H2O(l)����H1����C7H8(l)(Q)��9O2(g)=7CO2(g)��4H2O(l)����H2����
![]()
![]() ����H��+88.62 kJ��mol��1��������������ȷ����(����)
����H��+88.62 kJ��mol��1��������������ȷ����(����)
A. ��H1>��H2 B. NBD��������Q��������
C. NBD��Q�ȶ� D. NBDת��ΪQ�����ȷ�Ӧ
���𰸡�B
��������
A���٢�Ϊ���ȷ�Ӧ������H<0���Ƚ�ʱӦע�⡰������
B�����������غ���з�����
C������Խ�ͣ�����Խ�ȶ���
D�����������غ���з�����
A���٢�Ϊ���ȷ�Ӧ����H<0���۸÷�ӦΪ���ȷ�Ӧ����Q����������NBD�����á�H=����������������Ӧ�����������ó���H1>��H2��Ҳ���Բ��â٣��ڵó�����H1����H2=��H>0������H1>��H2����A˵����ȷ��
B�����ݢۣ��˷�Ӧ�����ȷ�Ӧ�����������غ㣬Q����������NBD����B˵������
C����������Խ�ͣ�����Խ�ȶ�������B�ķ�����NBD����������Q����NBD��Q�ȶ�����C˵����ȷ��
D�����ݢۡ�H>0��˵���˷�ӦΪ���ȷ�Ӧ����D˵����ȷ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����A��B��C��D��E��F����Ԫ�أ����ǵ������Ϣ���±���
Ԫ�ش��� | �����Ϣ |
A | �����ĵ������Ǵ�����������3�� |
B | ��ˮ�к�����һλ�Ľ���Ԫ�� |
C | L���1�����Ӻ��Ϊ�ȶ��ṹ |
D | ���������Ӻ�����18������ |
E | ʧȥһ�����Ӻ�ͳ�Ϊһ��ԭ�� |
F | ����Ϊ�����к����������� |
����д���пո�
��1��Aԭ�ӵĵ���ʽ��_______________________________��
��2��B���ӵĽṹʾ��ͼ��__________________________����B���������������������ͬ����������____________________________________��д�����֣��������ϱ�ʾ����
��3��CԪ�ص����ƣ�________��Cԭ����������ߵĵ���λ�ڵ�________�㡣
��4��D�Ķ��������ӵĵ���ʽ��___________________________��DԪ�ص�ij��ͬλ��ԭ��������Ϊ34����ԭ�Ӻ��ڵ�������Ϊ______________��
��5��A��E��F����Ԫ������γɶ����������ʣ��������ڹ��ۻ�����Ϊ________�����ӻ�����Ϊ________����д��һ�����ʼ��ɣ�