��Ŀ����
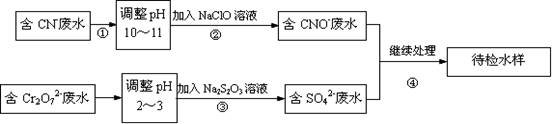
ij���ͭ�������ַ�ˮ��Ҫ������һ�ַ�ˮ�к���CN-���ӣ���һ�ַ�ˮ�к���Cr2O72-���ӣ��ó��ⶨ��ͼ��ʾ�ķ�ˮ�������̣�
�ش��������⣺
��1������������ˮ����������Ҫʹ�õķ�����
��2������ʹ�õ�NaClO��Һ�ʼ��ԣ������ӷ���ʽ����ԭ��
��3�����з�Ӧ��������ų����÷�Ӧ�����ӷ���ʽΪ
��4�����з�Ӧʱ��ÿ0.4molCr2O72-ת��2.4mol�ĵ��ӣ��÷�Ӧ�����ӷ���ʽΪ
��5��ȡ��������ˮ�����Թ��У��ȼ���NaOH��Һ���۲쵽����ɫ�������ɣ���������NaOH��Һ��ֱ�����ٲ�����ɫ����Ϊֹ���ټ���Na2S��Һ���к�ɫ�������ɣ�����ɫ�������٣�����ʹ�û�ѧ�����ϱ�Ҫ�����ֽ�����ԭ��

�ش��������⣺
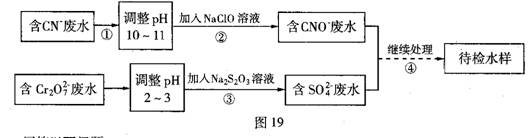
��1������������ˮ����������Ҫʹ�õķ�����
������ԭ��
������ԭ��
����2������ʹ�õ�NaClO��Һ�ʼ��ԣ������ӷ���ʽ����ԭ��
ClO-+H2O HClO+OH-
HClO+OH-
 HClO+OH-
HClO+OH-ClO-+H2O HClO+OH-
HClO+OH-
�� HClO+OH-
HClO+OH-��3�����з�Ӧ��������ų����÷�Ӧ�����ӷ���ʽΪ
CN-+ClO-�TCNO-+Cl-
CN-+ClO-�TCNO-+Cl-
����4�����з�Ӧʱ��ÿ0.4molCr2O72-ת��2.4mol�ĵ��ӣ��÷�Ӧ�����ӷ���ʽΪ
3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O
3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O
����5��ȡ��������ˮ�����Թ��У��ȼ���NaOH��Һ���۲쵽����ɫ�������ɣ���������NaOH��Һ��ֱ�����ٲ�����ɫ����Ϊֹ���ټ���Na2S��Һ���к�ɫ�������ɣ�����ɫ�������٣�����ʹ�û�ѧ�����ϱ�Ҫ�����ֽ�����ԭ��
����ˮ���л���Cu2+���Ӽ��Cu2++2OH-�TCu��OH��2�����ټ���Na2S��Һ��CuS��Cu��OH��2�����ܣ�����Cu��OH��2��s��+S2-��aq���TCuS��s��+2OH-��aq��
����ˮ���л���Cu2+���Ӽ��Cu2++2OH-�TCu��OH��2�����ټ���Na2S��Һ��CuS��Cu��OH��2�����ܣ�����Cu��OH��2��s��+S2-��aq���TCuS��s��+2OH-��aq��
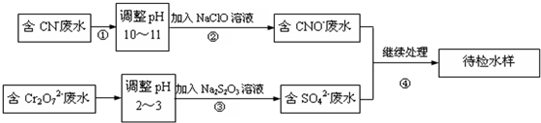
����������1����ת��ͼ��ͼ�е����ʿ�֪��NaClO��Cr2O72-���Ӷ����������ԣ���ʵ�����ӵ�ת��������������ԭ��Ӧʵ�ֵģ�
��2�������ε�ˮ������������Һ�Լ��Ե�ԭ��
��3��CN-���Ӿ��л�ԭ�ԡ�NaClO���������ԣ�����������ԭ����������ע���������������ж������
��4������ÿ0.4molCr2O72-ת��2.4mol�ĵ��������㱻��ԭ��CrԪ�صĻ��ϼۣ�����д���ӷ���ʽ��
��5������ͭ���������������ӷ�Ӧ���ɳ�����������ת����������
��2�������ε�ˮ������������Һ�Լ��Ե�ԭ��
��3��CN-���Ӿ��л�ԭ�ԡ�NaClO���������ԣ�����������ԭ����������ע���������������ж������
��4������ÿ0.4molCr2O72-ת��2.4mol�ĵ��������㱻��ԭ��CrԪ�صĻ��ϼۣ�����д���ӷ���ʽ��
��5������ͭ���������������ӷ�Ӧ���ɳ�����������ת����������
����⣺��1����ͼ��֪������ˮ�е�����ʵ������ת��ʱ��������������ԭ��Ӧ������Ҫʹ�õķ���Ϊ������ԭ�����ʴ�Ϊ��������ԭ����
��2��NaClO��Һ�ʼ��ԣ���������������ˮ���������������ӵ��µģ������ӷ�ӦΪClO-+H2O HClO+OH-���ʴ�Ϊ��ClO-+H2O
HClO+OH-���ʴ�Ϊ��ClO-+H2O HClO+OH-��
HClO+OH-��
��3�����������£�CN-������NaClO����������ԭ��Ӧ��������ų���������CNO-��Cl-���ӣ����ӷ�ӦΪCN-+ClO-�TCNO-+Cl-���ʴ�Ϊ��CN-+ClO-�TCNO-+Cl-��
��4��ÿ0.4molCr2O72-ת��2.4mol�ĵ��ӣ��軹ԭ��CrԪ�صĻ��ϼ�Ϊx����0.4mol��2����6-x��=2.4mol�����x=+3�������ӷ�ӦΪ3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
�ʴ�Ϊ��3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
��5����ͭ���������������ӷ�Ӧ����������ͭ������CuS��Cu��OH��2�����ܣ������Na2S��Һ�ܷ���������ת�����ʴ�Ϊ������ˮ���л���Cu2+���Ӽ��Cu2++2OH-�TCu��OH��2�����ټ���Na2S��Һ��CuS��Cu��OH��2�����ܣ�����Cu��OH��2��s��+S2-��aq���TCuS��s��+2OH-��aq����
��2��NaClO��Һ�ʼ��ԣ���������������ˮ���������������ӵ��µģ������ӷ�ӦΪClO-+H2O
 HClO+OH-���ʴ�Ϊ��ClO-+H2O
HClO+OH-���ʴ�Ϊ��ClO-+H2O HClO+OH-��
HClO+OH-�� ��3�����������£�CN-������NaClO����������ԭ��Ӧ��������ų���������CNO-��Cl-���ӣ����ӷ�ӦΪCN-+ClO-�TCNO-+Cl-���ʴ�Ϊ��CN-+ClO-�TCNO-+Cl-��
��4��ÿ0.4molCr2O72-ת��2.4mol�ĵ��ӣ��軹ԭ��CrԪ�صĻ��ϼ�Ϊx����0.4mol��2����6-x��=2.4mol�����x=+3�������ӷ�ӦΪ3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
�ʴ�Ϊ��3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
��5����ͭ���������������ӷ�Ӧ����������ͭ������CuS��Cu��OH��2�����ܣ������Na2S��Һ�ܷ���������ת�����ʴ�Ϊ������ˮ���л���Cu2+���Ӽ��Cu2++2OH-�TCu��OH��2�����ټ���Na2S��Һ��CuS��Cu��OH��2�����ܣ�����Cu��OH��2��s��+S2-��aq���TCuS��s��+2OH-��aq����
���������⿼������������ԭ��Ӧ��ʵ�ַ�ˮ��ת����ע���˻�ѧ��ʵ����������ϵ��ѧ��Ӧѧ���������ʵ����ʡ�Ԫ�صĻ��ϼۡ������غ�������

��ϰ��ϵ�д�
�����Ŀ