��Ŀ����
������ԭ��Ӧ�ڻ�ѧ��ҵ��Ӧ�ù㷺��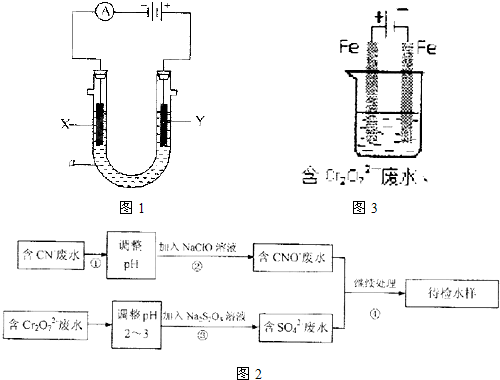
������ͼ1��ʾ��ʵ��װ�ã�
��1������ʯī�缫���NaHCO3��Һ��Y�缫�ϲ�����������
��2����Ҫ�����϶�ͭ����X�缫�IJ�����
��ij���ͭ�������ַ�ˮ��Ҫ������һ�ַ�ˮ�к���CN-���ӣ���һ�ַ�ˮ�к�Cr2O72-���ӣ��ó��ⶨ��ͼ2��ʾ�ķ�ˮ�������̣�
�ش��������⣺
��1������ʹ�õ�NaClO��Һ�ʼ��ԣ�ԭ����
��2�����з�Ӧʱ��ÿ0.4mol Cr2O72-ת��2.4mole-���÷�Ӧ�����ӷ���ʽΪ
��3��ȡ��������ˮ�����Թ��У��������NaOH��Һ������ɫ�������ɣ�����������ɫ����������Һ�м���Na2S��Һ���к�ɫ�������ɣ�����ɫ�������٣�����ʹ�û�ѧ�����ϱ�Ҫ������˵�����������
��ҵ���õ�ⷨ��������Cr2O72-��ˮ��
��1��Fe2+��������Һ�е�Cr2O72-��Ӧ�����ӷ���ʽ��
��2��ʵ����������ͼ3��ʾģ�����Cr2O72-�ķ�ˮ����ˮ�м�������NaCl��������Һ�ĵ����ԣ������������õ��������������������ƽ���ƶ�ԭ�����ͳ�������������������ԭ��
��3���õ�ⷨ��������Һ�� 0.01mol Cr2O72-ʱ���õ�������������

������ͼ1��ʾ��ʵ��װ�ã�
��1������ʯī�缫���NaHCO3��Һ��Y�缫�ϲ�����������
O2
O2
����2����Ҫ�����϶�ͭ����X�缫�IJ�����
��
��
��Y�缫��Ӧʽ��Cu-2e-=Cu2+
Cu-2e-=Cu2+
����ij���ͭ�������ַ�ˮ��Ҫ������һ�ַ�ˮ�к���CN-���ӣ���һ�ַ�ˮ�к�Cr2O72-���ӣ��ó��ⶨ��ͼ2��ʾ�ķ�ˮ�������̣�
�ش��������⣺
��1������ʹ�õ�NaClO��Һ�ʼ��ԣ�ԭ����
ClO-+H2O?HClO+OH-
ClO-+H2O?HClO+OH-
�������ӷ���ʽ��ʾ������2�����з�Ӧʱ��ÿ0.4mol Cr2O72-ת��2.4mole-���÷�Ӧ�����ӷ���ʽΪ
3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O
3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O
����3��ȡ��������ˮ�����Թ��У��������NaOH��Һ������ɫ�������ɣ�����������ɫ����������Һ�м���Na2S��Һ���к�ɫ�������ɣ�����ɫ�������٣�����ʹ�û�ѧ�����ϱ�Ҫ������˵�����������
����ˮ���л���Cu2+���Ӽ��Cu2++2OH-�TCu��OH��2�����ټ���Na2S��Һ��CuS��Cu��OH��2�����ܣ�����Cu��OH��2��s��+S2-��aq���TCuS��s��+2OH-��aq����
����ˮ���л���Cu2+���Ӽ��Cu2++2OH-�TCu��OH��2�����ټ���Na2S��Һ��CuS��Cu��OH��2�����ܣ�����Cu��OH��2��s��+S2-��aq���TCuS��s��+2OH-��aq����
����ҵ���õ�ⷨ��������Cr2O72-��ˮ��
��1��Fe2+��������Һ�е�Cr2O72-��Ӧ�����ӷ���ʽ��
Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O
Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O
����2��ʵ����������ͼ3��ʾģ�����Cr2O72-�ķ�ˮ����ˮ�м�������NaCl��������Һ�ĵ����ԣ������������õ��������������������ƽ���ƶ�ԭ�����ͳ�������������������ԭ��
������Ӧ������ˮ�е�H+��������ˮ�ĵ���ƽ�⣬�ٽ���ˮ�ĵ��룬ʹ��Һ��OH-Ũ��������Һ�ļ�����ǿ
������Ӧ������ˮ�е�H+��������ˮ�ĵ���ƽ�⣬�ٽ���ˮ�ĵ��룬ʹ��Һ��OH-Ũ��������Һ�ļ�����ǿ
����3���õ�ⷨ��������Һ�� 0.01mol Cr2O72-ʱ���õ�������������
8.48
8.48
g����������1�����̼��������Һ�����������������ӷŵ磬�����������ӷŵ磬ʵ�����൱�ڵ��ˮ��
��2�����ݵ�ƵĹ���ԭ��֪ʶ���ش�
��1�������ε�ˮ������������Һ�Լ��Ե�ԭ��
��2������ÿ0.4molCr2O72-ת��2.4mol�ĵ��������㱻��ԭ��CrԪ�صĻ��ϼۣ�����д���ӷ���ʽ��
��3������ͭ���������������ӷ�Ӧ���ɳ�����������ת����������
��1��Fe2+������Cr2O72-���ӷ���������ԭ��Ӧ����Fe3+���Ӻ�Cr3+���ӣ�
��2�����ŵ����У���Һ��c��H+�� ���٣�c��OH-��Ũ������
��3������Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��Cr3++3OH-�TCr��OH��3����Fe3++3OH-�TFe��OH��3�����м��㣻
��2�����ݵ�ƵĹ���ԭ��֪ʶ���ش�
��1�������ε�ˮ������������Һ�Լ��Ե�ԭ��
��2������ÿ0.4molCr2O72-ת��2.4mol�ĵ��������㱻��ԭ��CrԪ�صĻ��ϼۣ�����д���ӷ���ʽ��
��3������ͭ���������������ӷ�Ӧ���ɳ�����������ת����������
��1��Fe2+������Cr2O72-���ӷ���������ԭ��Ӧ����Fe3+���Ӻ�Cr3+���ӣ�
��2�����ŵ����У���Һ��c��H+�� ���٣�c��OH-��Ũ������
��3������Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��Cr3++3OH-�TCr��OH��3����Fe3++3OH-�TFe��OH��3�����м��㣻
����⣺��1��Y�缫Ϊ���������������������ӷŵ磺4OH--4e-=O2��+2H2O��
�ʴ�Ϊ��O2��
��2�����ʱ���Ʋ���������������ƽ���������������X�缫�IJ���������Y�缫�IJ�����ͭ���缫��ӦʽΪ��Cu-2e-=Cu2+��
�ʴ�Ϊ������Cu-2e-=Cu2+��
��1��NaClO��Һ�ʼ��ԣ���������������ˮ���������������ӵ��µģ������ӷ�ӦΪ��ClO-+H2O?HClO+OH-��
�ʴ�Ϊ��ClO-+H2O?HClO+OH-��
��2��ÿ0.4molCr2O72-ת��2.4mol�ĵ��ӣ��軹ԭ��CrԪ�صĻ��ϼ�Ϊx����0.4mol��2����6-x��=2.4mol�����x=+3�������ӷ�ӦΪ3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
�ʴ�Ϊ��3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
��3����ͭ���������������ӷ�Ӧ����������ͭ������CuS��Cu��OH��2�����ܣ������Na2S��Һ�ܷ���������ת����
�ʴ�Ϊ������ˮ���л���Cu2+���Ӽ��Cu2++2OH-�TCu��OH��2�����ټ���Na2S��Һ��CuS��Cu��OH��2�����ܣ�����Cu��OH��2��s��+S2-��aq���TCuS��s��+2OH-��aq����
��1������������Cr2O72-����������ԭ��Ӧ������ԭΪCr3+Ȼ������Cr��OH��3�������ظ��������ǿ�����ԣ��ܽ����ɵ�������������Ϊ���ۣ���6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O��
�ʴ�Ϊ��Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��
��2�����ŵ����У���Һ��c��H+�� ���٣�������ˮ�ĵ���ƽ�⣬�ٽ���ˮ�ĵ��룬ʹ��Һ��OH-Ũ��������Һ�ļ�����ǿ������Fe��OH��3��Cr��OH��3�������������������������ɳ�����ȫ��
�ʴ�Ϊ��������Ӧ������ˮ�е�H+��������ˮ�ĵ���ƽ�⣬�ٽ���ˮ�ĵ��룬ʹ��Һ��OH-Ũ��������Һ�ļ�����ǿ��
��3������Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��Cr3++3OH-�TCr��OH��3����Fe3++3OH-�TFe��OH��3��֪0.01mol Cr2O72-��������0.02molCr��OH��3��0.06molFe��OH��3�����ٵõ�������������0.02mol��103g/mol+0.06mol��107g/mol=8.48g��
�ʴ�Ϊ��8.48��
�ʴ�Ϊ��O2��
��2�����ʱ���Ʋ���������������ƽ���������������X�缫�IJ���������Y�缫�IJ�����ͭ���缫��ӦʽΪ��Cu-2e-=Cu2+��
�ʴ�Ϊ������Cu-2e-=Cu2+��
��1��NaClO��Һ�ʼ��ԣ���������������ˮ���������������ӵ��µģ������ӷ�ӦΪ��ClO-+H2O?HClO+OH-��
�ʴ�Ϊ��ClO-+H2O?HClO+OH-��
��2��ÿ0.4molCr2O72-ת��2.4mol�ĵ��ӣ��軹ԭ��CrԪ�صĻ��ϼ�Ϊx����0.4mol��2����6-x��=2.4mol�����x=+3�������ӷ�ӦΪ3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
�ʴ�Ϊ��3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
��3����ͭ���������������ӷ�Ӧ����������ͭ������CuS��Cu��OH��2�����ܣ������Na2S��Һ�ܷ���������ת����
�ʴ�Ϊ������ˮ���л���Cu2+���Ӽ��Cu2++2OH-�TCu��OH��2�����ټ���Na2S��Һ��CuS��Cu��OH��2�����ܣ�����Cu��OH��2��s��+S2-��aq���TCuS��s��+2OH-��aq����
��1������������Cr2O72-����������ԭ��Ӧ������ԭΪCr3+Ȼ������Cr��OH��3�������ظ��������ǿ�����ԣ��ܽ����ɵ�������������Ϊ���ۣ���6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O��
�ʴ�Ϊ��Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��
��2�����ŵ����У���Һ��c��H+�� ���٣�������ˮ�ĵ���ƽ�⣬�ٽ���ˮ�ĵ��룬ʹ��Һ��OH-Ũ��������Һ�ļ�����ǿ������Fe��OH��3��Cr��OH��3�������������������������ɳ�����ȫ��
�ʴ�Ϊ��������Ӧ������ˮ�е�H+��������ˮ�ĵ���ƽ�⣬�ٽ���ˮ�ĵ��룬ʹ��Һ��OH-Ũ��������Һ�ļ�����ǿ��
��3������Cr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��Cr3++3OH-�TCr��OH��3����Fe3++3OH-�TFe��OH��3��֪0.01mol Cr2O72-��������0.02molCr��OH��3��0.06molFe��OH��3�����ٵõ�������������0.02mol��103g/mol+0.06mol��107g/mol=8.48g��
�ʴ�Ϊ��8.48��
���������⿼���˵��ԭ����������ԭ��Ӧ��ע���˻�ѧ��ʵ����������ϵ��ѧ��Ӧѧ���������ʵ����ʡ�Ԫ�صĻ��ϼۡ������غ�������

��ϰ��ϵ�д�
�����Ŀ

 ��д��������������Һ��������������Ӧ����
��д��������������Һ��������������Ӧ����
 2NH3(g)����H= -92.4 kJ/mol��
2NH3(g)����H= -92.4 kJ/mol��