��Ŀ����
����Ŀ����֪�Ͽ�1 mol Cl2(g)��Cl��Cl����Ҫ����243 kJ���������������仯ʾ��ͼ������˵�����Ȼ�ѧ����ʽ��ȷ����

A.H2(g)+Cl2(g)=2HCl(g) ��H=��185 kJ��mol��1
B.����1 mol H2(g)�е�H��H���ų�121.5 kJ����
C.�Ͽ�1 mol HCl(g)�е�H��Cl��Ҫ����864 kJ����
D.HCl(g)=![]() H2(g)+
H2(g)+![]() Cl2(g) ��H=��92.5 kJ��mol��1
Cl2(g) ��H=��92.5 kJ��mol��1
���𰸡�A
��������
A. ��ͼ��֪H2(g)+Cl2(g)=2HCl(g) ��H=-(864-679) kJ/mol=-185 kJ/mol��A��ȷ��
B. ��ͼ��֪�Ͽ�1 mol H2(g)�е�H��H����1 mol Cl2(g)�е�Cl��Cl��������679 kJ/mol������������֪�Ͽ�1 mol Cl2(g)��Cl��Cl����Ҫ����243 kJ���������ԶϿ��Ͽ�1 mol H2(g)�е�H��H����Ҫ���յ�����Ϊ679 kJ-243 kJ=436 kJ���������1 mol H2(g)�е�H��H���ų�436 kJ������B����
C. ����2 mol HCl (g)�е�H-Cl���ų�864 kJ����������������2 mol HCl (g)�е�H-Cl���ų�432 kJ����������Ͽ�1 mol HCl(g)�е�H��Cl��Ҫ����432 kJ������C����
D. ����ѡ��A������֪2 mol HCl(g)��Ӧ����1 mol H2(g)��1 mol Cl2(g)����185 kJ�����������1 mol HCl��Ӧ����H2(g)��Cl2(g)����92.5 kJ���������Ȼ�ѧ����ʽΪHCl(g)=![]() H2(g)+
H2(g)+![]() Cl2(g) ��H=+92.5 kJ��mol��1��D����
Cl2(g) ��H=+92.5 kJ��mol��1��D����
�ʺ���ѡ����A��

����Ŀ����֪1g������ȫȼ������Һ̬ˮʱ�ų�����143kJ,18gˮ�������Һ̬ˮ�ų�44kJ����������������������:
O=O | H��H | H��O(g) | |
����/(kJ��mol-1) | 496 | 436 | x |
�����xΪ
A.920B.557C.463D.188
����Ŀ��X��Y��Z��M��Q��R��6�ֶ�����Ԫ�أ���ԭ�Ӱ뾶����Ҫ���ϼ����£�
Ԫ�ش��� | X | Y | Z | M | Q | R |
ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.102 | 0.075 | 0.077 | 0.037 |
��Ҫ���ϼ� | ��2 | ��3 | ��6����2 | ��5����3 | ��4����4 | ��1 |
��1��Z��Ԫ�����ڱ��е�λ����_________________________��
��2��Ԫ��Q��R�γɵĻ�����A�ǹ�ʵ���������A�Ʊ��Ҵ��Ļ�ѧ����ʽ��_______________________��
��3������ͭ��Ԫ��M������������Ӧˮ�����Ũ��Һ������Ӧ�����ӷ���ʽΪ___________________��
��4��Ԫ��X�Ľ����Ա�Ԫ��Y______���ǿ������������
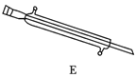
��5��Ԫ��Q��Ԫ��Z�ĺ���Ӱ��������ܣ�������ͼװ��A�ڸ����½�������Ԫ��Q��Ԫ��Zת��ΪQO2��ZO2��

������a�ijɷ���________________���ѧʽ����
����������Ԫ��Z��FeZ����ʽ���ڣ���A�з�Ӧ����ZO2���ȶ��ĺ�ɫ�������Ӧ�Ļ�ѧ����ʽ��_________________________________��
����Ŀ������ʵ���������������۶�Ӧ��ϵ��ȷ����
ѡ�� | ʵ����� | ʵ������ | ���� |
A | ��ͬ�¶��£�ͬʱ�� ��4 mL 0.1 molL-1 KMnO4������Һ����4 mL 0.2 molL-1 KMnO4������Һ�У��ֱ����4 mL 1 molL-1 H2C2O4��Һ | ������Һ����ɫ | ��ʵ�������£�KMnO4Ũ��ԽС����Ӧ����Խ�� |
B | ��ú¯�����ȵ�ú̿��������ˮ | ��������ɫ���棬ú̿ȼ�ո��� | ������ˮ��ʹú̿ȼ�շų���������� |
C | ����2NO2(g) | ��ɫ���� | ֤������Ӧ�Ƿ��ȷ�Ӧ |
D | �ֱ�ⶨ�����µ����ʵ���Ũ�ȵ�Na2SO3��Na2CO3��Һ��pH | ���߽ϴ� | ֤���ǽ����� S��C |
A.AB.BC.CD.D