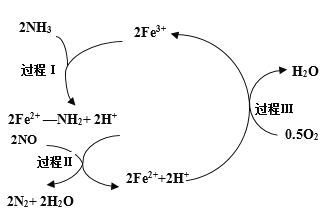
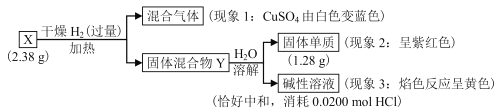
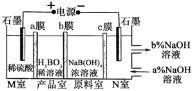
��Ŀ����
����Ŀ����������Ҫ�Ļ���ԭ�ϣ���ҵ����N2��H2��һ�������ºϳɡ�
(1)��֪H2�ı�ȼ����Ϊ��H1����akJ��mol��1��NH3�ı�ȼ����Ϊ��H2����bkJ��mol��1����ϳɰ���Ӧ��N2(g)��3H2(g)![]() 2NH3(g)�ġ�H3��_________ kJ��mol-1 (�ú�a��b�Ĵ���ʽ��ʾ)��
2NH3(g)�ġ�H3��_________ kJ��mol-1 (�ú�a��b�Ĵ���ʽ��ʾ)��
(2)�ϳɰ���Ӧ��N2(g)��3H2(g) ![]() 2NH3(g)��һ���¶������Է����У����H3______0(�>����<��)��ԭ����__________��
2NH3(g)��һ���¶������Է����У����H3______0(�>����<��)��ԭ����__________��
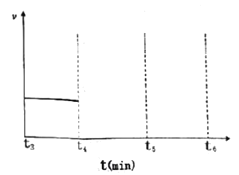
(3)һ���¶��£������Ϊ2L���ܱ�������ͨ��һ������N2��H2������Ӧ���й����ʵ����淴Ӧʱ��ı仯��ͼ��ʾ�����¶��£���Ӧ��ƽ�ⳣ��Ϊ_________________��
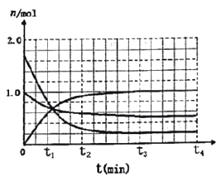
����t4ʱ�̣�ͬʱ����ϵ����ͨ��0.5 mol N2��1moNH3��t5ʱ�����´ﵽƽ�⣬������ͼ�л���t4~t6ʱ����淴Ӧ������ʱ��ı仯ͼ_____________��
(4)���ױ�������ѹ�°��������ú���ϡ�͵ĵ����ֱ�ͨ��һ�����ȵ�570��ĵ����У����ø����ӵ����Ե�SCY�մ�(�ܴ���H��)Ϊ���ʿ��ڵ缫��ֱ�����ɰ����������ķ�Ӧ����ʽΪ___________��
���𰸡�-3a+2b < �˷�Ӧ���Ǽ��ټ���S<0��Ҫ�ô˷�Ӧ��������H<0 1000 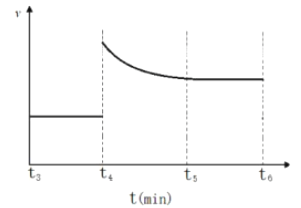
![]()
��������
��1����֪H2�ı�ȼ����Ϊ��H1����akJ��mol��1��NH3�ı�ȼ����Ϊ��H2����bkJ��mol��1����д����Ӧ���Ȼ�ѧ����ʽ![]() ��
��![]() �� ��Ҫ�õ�N2(g)��3H2(g)
�� ��Ҫ�õ�N2(g)��3H2(g)![]() 2NH3(g)�����ݸ�˹���ɣ���H3��-3a+2b��
2NH3(g)�����ݸ�˹���ɣ���H3��-3a+2b��
��2���ϳɰ���Ӧ��һ���¶������Է����У���Ӧ���ȣ���H3��0���˷�Ӧ���Ǽ��ټ���S<0��Ҫ�ô˷�Ӧ��������H<0��
��3������ͼ���֪��ƽ��ʱ������ʣ��0.2mol������ʣ��0.5mol������ʣ��1mol��ƽ�ⳣ��Ϊ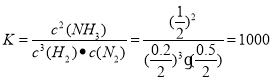 ������t4ʱ�̣�ͬʱ����ϵ����ͨ��0.5 mol N2��1moNH3����ʱ��
������t4ʱ�̣�ͬʱ����ϵ����ͨ��0.5 mol N2��1moNH3����ʱ��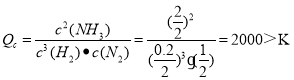 ��ƽ��������У��ɵõ�ͼ��
��ƽ��������У��ɵõ�ͼ�� 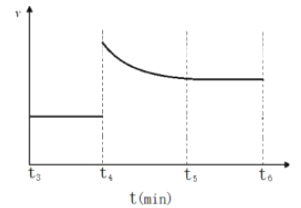 ��
��
��4������������ԭ��Ӧ������ǵ����ŵ磬�缫����ʽΪ![]() ��
��

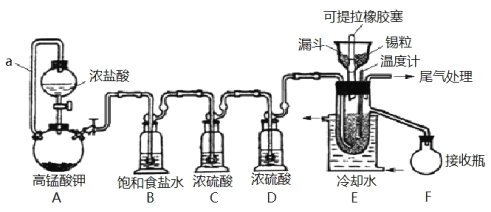
����Ŀ��ʵ���ҿ��������������Ʊ���ˮ���Ȼ�����SnCl4����SnCl4�ӷ���������ˮ�⣬Cl2��������SnCl4���Ʊ�ԭ����ʵ��װ��ͼ��ͼ��
Sn(s)+2Cl2(g)=SnCl4(l) ��H=�C511kJmol-1
�����õ����й��������£�
���� | Sn | SnCl4 | CuCl2 |
�۵�/�� | 232 | -33 | 620 |
�е�/�� | 2260 | 114 | 993 |
�Ʊ������У����������ģ�������������ʱ��Ӧ���в�����������SnCl4Һ��������ڸ߶�ʱ��Һ̬���ᆳ����������ƿ���ش��������⣺
��1��a��������___��
��2��A�з�Ӧ�Ļ�ѧ����ʽ��___��
��3��B��������___��
��4��E����ȴˮ��������___��
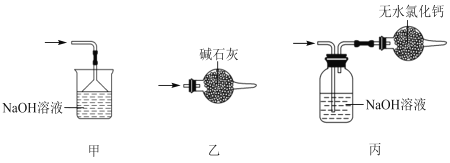
��5��β������ʱ����ѡ�õ�װ����___������ţ���
��6�������к�ͭ������ E �в��� CuCl2������Ӱ�� F �в�Ʒ�Ĵ��ȣ�ԭ����___��
��7��SnCl4��Ʒ�к���Cl2������ʱ����������м������ɵô�����SnCl4����������в���Ҫ�õ���������___(�����)
A����Һ©�� B���¶ȼ� C������ƿ D�������� E��������ƿ